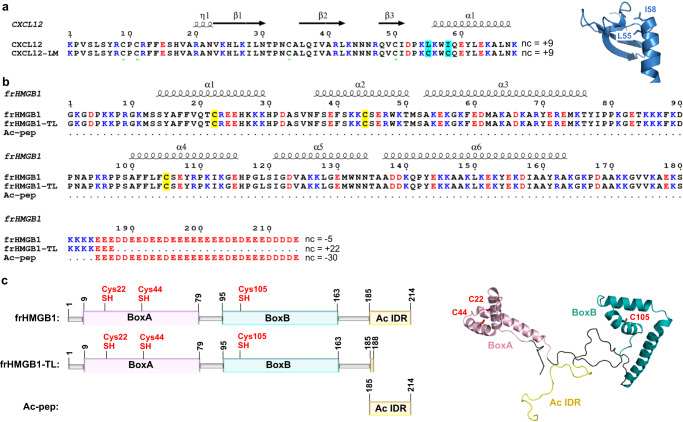
Fig. 1. CXCL12 and frHMGB1 constructs.
Amino acid sequence of: (a) CXCL12 and locked monomer CXCL12 mutant L55C/I58C (CXCL12-LM) and (b) frHMGB1, frHMGB1-TL and Ac-pep. Basic and acidic residues are shown in blue and red, respectively, nc indicates the net charge. L55C and I58C are colored in cyan on CXCL12 sequences. C22, C44 and C105 are colored in yellow on HMGB1 sequences. On the top of the alignments the elements of secondary structures are indicated. In (a) on the right is reported the cartoon representation of CXCL12 (pdb code: 2KEE), with L55 and I58 explicitly shown in sticks. c Schematic diagram of the fully reduced form of HMGB1 (frHMGB1), tail-less frHMGB1 (frHMGB1-TL), the acidic peptide (Ac-pep) corresponding to the HMGB1 acidic intrinsically disordered region (Ac IDR). BoxA (pink), BoxB (cyan) and Ac IDR (yellow) are represented with boxes and colored on frHMGB1 structure (AF-P63159). The side chains of fully reduced cysteines are represented in red sticks.