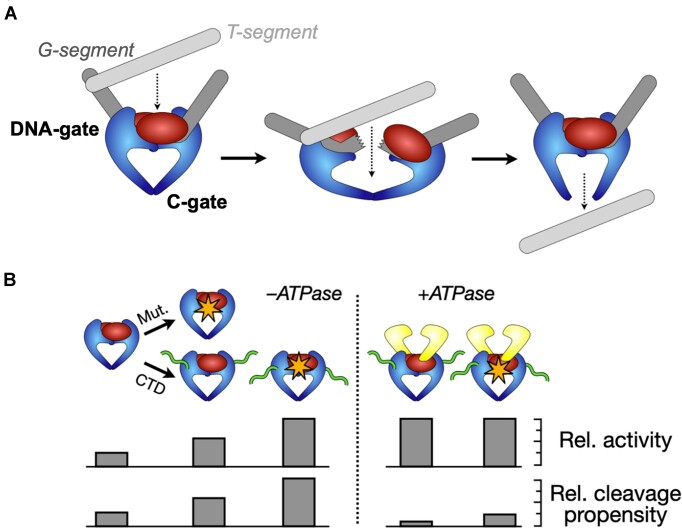
Figure 6.
Schematic depicting the action of a hypothetical, damage-prone ancestral type II topoisomerase before the acquisition of a regulating ATPase element for mitigating unwarranted DNA breakage. (A) An ancestral, ATPase-less type II topoisomerase could perform strand passage by a ‘two-gate’ mechanism that relies on just the DNA- and C-gates of the enzyme. Data reported here for wildtype and mutant (R757W) hTOP2β show that this minimal type II topoisomerase construct possesses such an activity. (B) Acquisition of a DNA binding C-terminal domain (CTD) and/or mutations that destabilize subunit interfaces can potentially lead to enhanced activity but also to an enhanced propensity for cleavage. The acquisition of the GHKL family ATPase domains substantially reduces deleterious DNA cleavage activity.