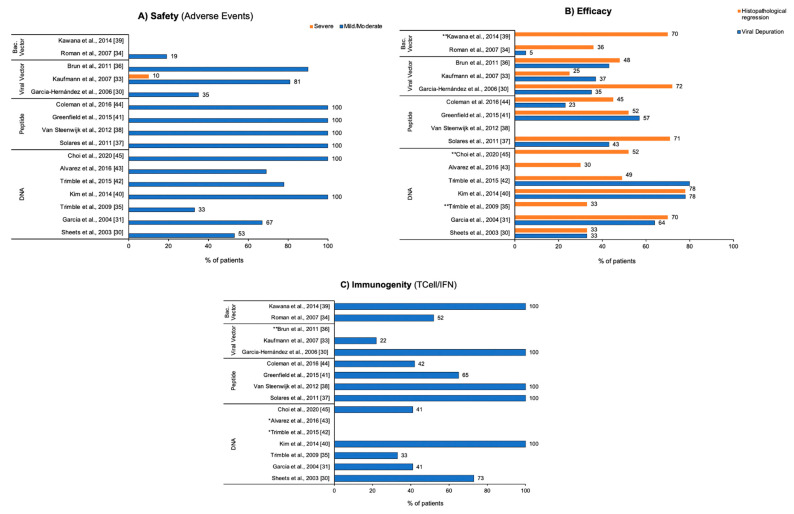
Figure 3.
Efficacy, immunogenicity, and safety of the therapeutic vaccine used in the studies. The most frequent parameters in the studies are expressed in % of patients who presented them. (A) Panel Safety compares mild/moderate and severe adverse events, considering the % of patients presenting the most frequent events of the category. (B) Panel Efficacy compares viral reduction and histological regression. (C) Panel Immunogenicity compares Tcell/IFN responses. * Data presented in the study did not allow precise percentage descriptions. ** Parameter not evaluated in the study.