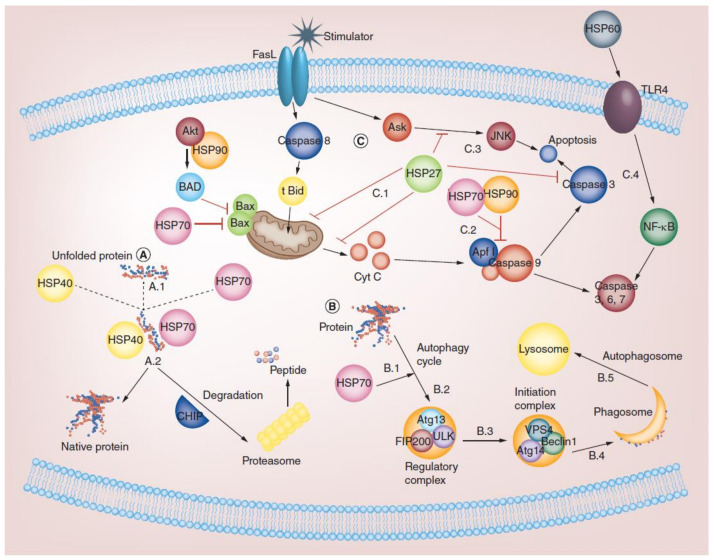
Figure 1.
Role of HSP in biochemical functions of cells. (A) Chaperone activity: misfolded proteins that accumulate in the cytosol bind to HSPs to maintain homeostatic balance (A.1). For example, the HSP70/HSP40 complex guides the degradation of misfolded proteins into short peptides via proteasome (A.2). (B) Role of HSP in the autophagy pathway: the autophagy cycle begins by binding HSP70 to the protein (B.1). Regulation of Atg13, FIP200, and ULK1 complex induced by HSPs (B.2). The initiation of the VPS4, Atg14, and Beclin1 complexes is regulated by HSPs (B.3). Ultimately, phagosomes are formed using phagosomal markers (B.4). Finally, autophagosomes or amphisomes fuse with lysosomes (B.5). (C) Role of HSP in different apoptotic pathways: HSPs help in modulating pro-apoptotic signals through FasL at the mitochondrial and post-mitochondrial levels. HSP70 and HSP27 appear to suppress the release of mitochondrial pro-apoptotic proteins by activating Bax and tBid (truncated Bid), respectively (C.1). HSP27, HSP70, and HSP90 can interact with and recruit Apaf-1 (apoptosis protease-activating factor 1) by directly sequestering cytochrome C to prevent apoptosome oligomerization and activation (C.2). HSP27 can also inhibit apoptosis via inactivation of caspase 3 and ASK (apoptosis signal-regulated kinase) (C.3). Furthermore, HSP60 and TLR-4 interactions mediate the NF-kappaB (NF-κB) signaling pathway, resulting in the activation of caspases 3, 6, and 7 and DNase (C.4). Reprinted (adapted) with permission from Ref. [44]. Copyright 2019, Future Medicine Ltd., London, UK.