Figure 5.
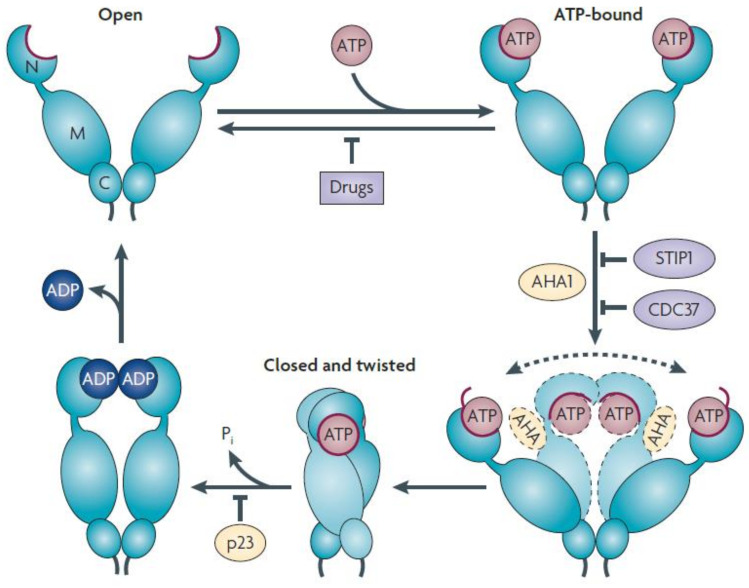
The chaperone, HSP90, undergoes multiple conformational states in the absence of nucleotides or other factors. ATP binding and hydrolysis shift conformational equilibrium by lowering the energy barrier between specific conformations, thus providing a conformational cycle for HSP90 [165]. ATP binds to the undimerized NTD (open state) of HSP90 and leads to N-terminal dimerization caused by the “lid” segment (red) repositioning. The subsequent structural rearrangement establishes a (closed and twisted) conformation of HSP90, which is suitable for ATP hydrolysis. The co-chaperone AHA1 increases HSP90 ATPase 1 activity by promoting conformational changes required to achieve ATPase competence. In the absence of AHA1, it was very difficult for HSP90 to achieve ATPase-competent conformation (dotted arrow). A number of co-chaperones, including Sti1p (p60HOP), Cdc37 homolog (cell division cycle 37), and N-domain-binding HSP90 inhibitors, prevent dimerization of the N-terminal domain. PTGES3 (prostaglandin E synthase 3 cytosolic, so-called p23 protein) deregulates the ATPase cycle and stabilizes its closed conformation. Reprinted (adapted) with permission from Ref. [163]. Copyright 2010, Springer Nature.