Abstract
A high-yield 3-methylthiopropanol (3-Met) yeast Y1402 was obtained from sesame-flavored Daqu, and it was identified as Saccharomycopsis fibuligera. S. fibuligera Y1402 showed a broad range of growth temperatures and pH, as well as the maximum tolerance to glucose, NaCl, nicotine, and 3-Met at 50% (w/w), 15% (w/v), 1.2 g/L, and 18 g/L, respectively. After optimization using single-factor experiments, a Plackett–Burman design, a steepest ascent test, and a Box–Behnken design, the 3-Met yield reached 4.03 g/L by S. fibuligera Y1402 under the following optimal conditions: glucose concentration of 40 g/L, yeast extract concentration of 0.63 g/L, Tween 80 concentration of 2 g/L, L-methionine concentration of 5 g/L, liquid volume of 25 mL/250 mL, initial pH of 5.3, fermentation temperature of 32 °C, inoculum size of 0.8%, shaking speed of 210 rpm, and fermentation time of 54 h. The fermentation was scaled up to a 3 L fermenter under the optimized conditions, and the yield of 3-Met reached 0.71 g/L. Additionally, an aroma analysis revealed that the flavor substances produced by S. fibuligera Y1402 in sorghum hydrolysate medium was mainly composed of compounds with floral, sweet, creamy, roasted nut, and clove-like aromas. Therefore, S. fibuligera has great potential for application in the brewing of Baijiu and other fermented foods.
Keywords: Saccharomycopsis fibuligera, Baijiu, tolerance, fermentation condition optimization, 3-methylthiopropanol, aroma characteristics
1. Introduction
3-Methylthiopropanol (3-Met) is a highly important sulfur-containing flavor compound that exhibits a low aroma threshold, presenting a pleasant meaty, grilled cheese, or sweet aroma at concentrations ranging between 1 and 3 mg/L [1,2,3]. It is a widely used edible flavor compound, and its market demand is growing rapidly [1]. Owing to its cheap cost, chemical synthesis is now the primary method for producing 3-Met; however, this synthesis has disadvantages as well, such as the use of hazardous raw materials, pollution during the synthesis process, and challenges in completely removing harmful byproducts [4]. These problems cannot meet the increasing consumer demand for environmentally friendly, green, healthy, and natural flavorings [4,5]. In this context, research efforts have shifted to the biotransformation synthesis, an ecologically safe, effective, and green approach for producing 3-Met [6]. Future predictions indicate that it will take precedence over other methods for the commercial synthesis of natural 3-Met.
At present, there are only a small number of natural microbial species that are involved in the synthesis of 3-Met. Only a few bacteria such as Oenococcus oeni, Lactobacillus paracasei, and Lactobacillus curvatus, and yeasts, such as Saccharomyces cerevisiae, Kluyveromyces lactis, and Debaryomyces hansenii, have been reported to produce 3-Met, but their yield was relatively low [1,4,7,8,9,10]. Except for S. cerevisiae SC408, S. cerevisiae SC57, S. cerevisiae S288C, K. lactis KL71, and the recently studied Hyphopichia burtonii YHM-G, the yield of 3-Met by other natural microorganisms were below 0.5 g/L [7,11,12,13,14]. To enhance the production of 3-Met by microorganisms, scientists have employed molecular biology techniques to examine the essential genes and enzymes responsible for 3-Met synthesis in native strains [2,15]. To maximize the output of 3-Met synthesis, these naturally existing strains were modified by overexpressing or shutting down genes, such as the aminotransferase genes ARO8 and ARO9 and the decarboxylase gene ARO10 [14,15,16,17]. The greatest 3-Met output recorded in prior research was similarly attained by the genetically engineered strain, with a level of 4.38 g/L [18].
Even though genetically modified strains offer several potential benefits for the industrial production of 3-Met, consumers in some countries still do not consider them as natural products [7]. Consequently, there are some restrictions on how they may be used. For instance, 3-Met is a flavoring ingredient that plays a major role in the quality of many fermented foods, including cheese, wine, beer, soy sauce, Baijiu, and Huangjiu [8,19,20]. Research has shown that the 3-Met in these fermented foods mainly came from the microbial biotransformation, and its content has depended on the metabolic activity of microorganisms [19]. However, due to certain national policies, the use of genetically modified strains in the production and processing of fermented foods is strictly restricted [7]. Therefore, in order to improve the content of 3-Met in fermented foods, it is required by current rules to investigate and evaluate the fermentation properties of naturally occurring strains that produce large levels of 3-Met. Through controlling the metabolic processes of these indigenous microbes, it is feasible to elevate the 3-Met concentration and enhance the flavor profile of fermented foods [1,19]. Consequently, it is essential to identify and obtain more natural strains from various fermented food brewing conditions that provide elevated levels of 3-Met.
In this study, a high-yield 3-Met-producing strain was screened from the traditional Baijiu brewing process using traditional screening methods. The growth characteristics and fermentation conditions for 3-Met production were investigated. This study aims to increase the 3-Met content in Baijiu, hence improving its overall quality. In addition, this study will offer valuable strains and theoretical frameworks applicable to related fields.
2. Materials and Methods
2.1. Materials, Reagents, and Media
Sesame-flavored high-temperature Daqu (a starter for Baijiu production) at different production stages were provided by Guojing Group, Shandong, China Bandaojing Co., Ltd. (Zibo, China). 3-Met, L-methionine, and chromatography-grade methanol were purchased from Sigma-Aldrich (St. Louis, Mo, USA). Other chemical reagents were of analytical grade and can be commercially obtained unless otherwise stated. Luria–Bertani medium (LB medium for bacteria); yeast extract peptone dextrose medium (YPD medium for yeast) and potato dextrose agar medium (PDA medium for mold) for screening and culturing strains; Wallerstein laboratory nutrient agar medium (WL medium) for strain morphology identification; sorghum hydrolysate medium (SHM) for aroma compounds production; and original fermentation medium for 3-Met production were prepared as described previously [7,21,22].
2.2. Screening for Strain with High Yield of 3-Met
The isolation of strains was performed according to Fan et al. using the traditional plate coating method with LB, YPD, and PDA medium [23]. All isolated strains were individually cultured in their respective liquid culture media at 30 °C and 200 rpm. Bacteria and yeast were cultured for 36 h, while molds were cultured for 60 h. The cultured strains were adjusted to a cell or spore count of 1 × 106 per milliliter using a hemocytometer. The process of producing 3-Met included transferring 0.1 mL of the corrected culture to a 250 mL shake flask with 50 mL of the original fermentation medium and incubating it for 48 h at 30 °C at 200 rpm, as described by Ma et al. [7]. Each strain was subjected to three parallel experiments. Strains showing the highest yield of 3-Met were selected for further research.
2.3. Identification and Biochemical Characteristics of S. fibuligera Y1402
2.3.1. Morphological Observation
Colony Morphology Observation: The yeasts were inoculated in YPD liquid culture medium and incubated for 24 h. The activated strains were streaked onto WL medium using the three-zone streaking method and incubated at 30 °C for 48 h in a constant humidity incubator. After incubation, colony morphology was observed.
Cell Morphology Observation: A drop of sterile water was placed in the center of a sterilized microscope slide. Using an inoculating loop, a small amount of yeast cells from a single colony on the WL medium was picked and evenly spread in the sterile water on the slide. The cells were fixed by heating over an alcohol flame and were stained with methylene blue. A coverslip was placed on top, excess stain was removed using absorbent paper, and the slide was observed under an electron microscope for cell morphology observation.
2.3.2. Physiological and Biochemical Identification
Physiological and biochemical tests were conducted by following the protocols outlined and described by Buchana et al. and Dong et al. [24,25]. These tests included carbon source fermentation, carbon source assimilation, nitrogen source assimilation, starch hydrolysis test, a methyl red test, an indole test, a urea test, a hydrogen sulfide test, a litmus milk test, a Voges–Proskauer test, a gelatin liquefaction test, and a citrate test.
2.3.3. Molecular Biology Identification
Cultivation and Collection of Yeast Cells: The activated yeast cells were inoculated into YPD liquid medium and incubated at a temperature of 28 °C and a speed of 180 rpm for 48 h. The cells were then collected by centrifugation at 13,751× g for 10 min.
Extraction of Yeast Genomic DNA and Amplification of 26S rDNA: The extraction of yeast genomic DNA followed the instructions of a fungal DNA extraction kit. The extracted yeast total DNA was used as a template for amplifying the 26S rDNA D1/D2 region using the universal primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′). The PCR reaction mixture consisted of 2.5 μL of LA PCR buffer, 1 μL of each primer, 2 μL of dNTP, 0.2 μL of LAtaq enzyme, 2 μL of DNA, and ddH2O to a final volume of 25 μL. The PCR amplification program was as follows: initial denaturation at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1 min for a total of 30 cycles, followed by a final extension at 72 °C for 10 min. The PCR products of yeast DNA were analyzed by electrophoresis on a 1% agarose gel.
Sequencing and Homology Alignment: The purified 26S rDNA D1/D2 amplification products were sequenced to obtain the original sequences of the PCR fragments. The sequence alignment software BioEdit 7.0.9 (Borland Software Corporation, Scotts Valley, CA, USA) was used to align the sequences with the forward sequence. The corrected 26S rDNA D1/D2 region sequences were then compared with the corresponding sequences of known yeast species in the GenBank nucleotide sequence database using a BLAST search to determine the similarity.
2.3.4. Determination of S. fibuligera Performance
After being activated, S. fibuligera Y1402 was introduced into YPD medium with a 0.2% inoculum size to assess its growth temperature (ranging from 20 °C to 50 °C), pH levels (ranging from 1 to 14), sugar tolerance (33.3%, 37.5%, 41.2%, 44.4%, 47.4%, 50%, and 52.4%, w/w), NaCl tolerance (0%, 3%, 6%, 9%, 12%, 15%, 18%, and 21%, w/v), ethanol tolerance (0%, 3%, 6%, 9%, 12%, 15%, 18%, and 21%, v/v), nicotine tolerance (0.3 g/L, 0.4 g/L, 0.6 g/L, 0.7 g/L, 1.0 g/L, and 1.2 g/L), and 3-Met tolerance (ranging from 5% to 20%). The cultures were incubated in a shaking incubator at 28 °C and 180 rpm for 48 h. The OD560 was measured using a turbidity method. A blank control was performed using uninoculated medium under various cultivation conditions.
2.3.5. Production of Aromatic Compounds
The activated S. fibuligera Y1402 were inoculated at a 0.2% inoculum size into the SHM. The cultures were incubated in a shaking incubator at a temperature of 28 °C and a shaking speed of 180 rpm for 48 h. The fermentation broth was then centrifuged at 4 °C and 13,751× g for 10 min. The supernatant was filtered through a 0.22 µm filter membrane and analyzed using headspace solid-phase microextraction–gas chromatography–mass spectrometry (HS-SPME–GC–MS) to detect the volatile aroma compounds produced by the yeast. A control group devoid of yeast cells was used. The previously disclosed method was used to treat the SHM medium.
2.4. Optimization of Fermentation Conditions for 3-Met Production
2.4.1. Single-Factor Experiment
Based on the screening conditions mentioned above, a single-factor experiment was conducted to investigate the effects of medium composition (glucose concentration, yeast extract concentration, surfactant type and concentration, L-methionine concentration) and fermentation conditions (L-methionine addition time, temperature, initial pH, inoculum size, shaking speed, liquid volume, fermentation time) on the production of 3-Met by S. fibuligera Y1402 (Table S1).
2.4.2. Plackett–Burman Design
Nine factors (glucose concentration (A), yeast extract concentration (B), L-methionine concentration (C), temperature (D), initial pH (E), fermentation time (F), inoculum size (G), liquid volume (H), and shaking speed (I)) were chosen for optimization using the Plackett–Burman experimental design based on the significant difference analysis of the results of the single-factor experiment and experience. Each factor was tested at two levels, high (+1) and low (−1), and the experimental design was conducted using Design-Expert 11 software (Table 1).
Table 1.
Plackett–Burman design for evaluating factors influencing 3-Met production and the statistical analysis.
| NO. | Factors | Y: 3-Met Concentration (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A: Glucose Concentration (g/L) | B: Yeast Extract Concentration (g/L) | C: L-Methionine Concentration (g/L) | D: Temperature (°C) | E: Initial pH | F: Fermentation Time (h) | G: Inoculum Size (%) | H: Liquid Volume (mL/250 mL) | I: Shaking Speed (rpm) | ||
| 1 | 50 (+1) | 0.4 (−1) | 6 (+1) | 36 (+1) | 4.5 (−1) | 60 (+1) | 0.4 (−1) | 12.5 (−1) | 180 (−1) | 2.34 |
| 2 | 30 (−1) | 0.4 | 2 (−1) | 28 (−1) | 4.5 | 48 (−1) | 0.4 | 12.5 | 180 | 0.50 |
| 3 | 40 (0) | 0.6 (0) | 4 (0) | 32 (0) | 5 (0) | 54 (0) | 0.8 (0) | 25 (0) | 225 (0) | 3.65 |
| 4 | 30 | 0.4 | 6 | 36 | 5.5 (+1) | 48 | 1.2 (+1) | 37.5 (+1) | 180 | 2.45 |
| 5 | 50 | 0.4 | 2 | 28 | 5.5 | 60 | 1.2 | 12.5 | 270 (+1) | 2.74 |
| 6 | 30 | 0.4 | 2 | 36 | 5.5 | 60 | 0.4 | 37.5 | 270 | 2.75 |
| 7 | 40 | 0.6 | 4 | 32 | 5 | 54 | 0.8 | 25 | 225 | 3.81 |
| 8 | 50 | 0.4 | 6 | 28 | 4.5 | 48 | 1.2 | 37.5 | 270 | 2.13 |
| 9 | 50 | 0.4 | 2 | 36 | 4.5 | 48 | 0.4 | 37.5 | 270 | 1.90 |
| 10 | 40 | 0.4 | 4 | 32 | 5 | 54 | 0.8 | 25 | 225 | 3.83 |
| 11 | 50 | 0.8 (+1) | 6 | 28 | 5.5 | 60 | 0.4 | 37.5 | 180 | 2.73 |
| 12 | 30 | 0.8 | 6 | 28 | 5.5 | 48 | 0.4 | 12.5 | 270 | 2.89 |
| 13 | 30 | 0.8 | 2 | 28 | 4.5 | 60 | 1.2 | 37.5 | 180 | 2.32 |
| 14 | 50 | 0.8 | 2 | 36 | 5.5 | 48 | 1.2 | 12.5 | 180 | 2.96 |
| 15 | 30 | 0.8 | 6 | 36 | 4.5 | 60 | 1.2 | 12.5 (−1) | 270 | 3.54 |
| Effect (Exi) | 0.00291 | 0.712 | 0.121 | 0.0548 | 0.631 | 0.02493 | 0.632 | −0.00459 | 0.00494 | |
| F values | 0.19 | 18.54 | 13.38 | 10.96 | 22.7 | 20.43 | 14.59 | 0.75 | 11.29 | |
| p values | 0.683 | 0.013 | 0.022 | 0.03 | 0.009 | 0.011 | 0.019 | 0.435 | 0.028 | |
| Rank | 9 | 3 | 5 | 7 | 1 | 2 | 4 | 8 | 6 | |
| Significance | - | * | * | * | ** | * | * | - | * | |
Note: “-”, not significant (p > 0.05); “*”, significant at the 5% level (p < 0.05); “**”, significant at the 1% level (p < 0.01).
2.4.3. Steepest Ascent Test
Seven factors (initial pH, fermentation time, yeast extract concentration, inoculum size, L-methionine concentration, shaking speed, and temperature) that had a significant impact on 3-Met production by S. fibuligera Y1402, as determined from the Plackett–Burman experiment, were selected for optimization using the steepest ascent test. The direction of change for each factor was based on its correlation with 3-Met production by S. fibuligera Y1402 in the Plackett–Burman design. The gradient for each factor was determined based on the principles of the steepest ascent test, single-factor experiment results, and experimental experience. A total of five experimental gradients were set up (Table 2).
Table 2.
Experimental designs and results of the steepest ascent test for 3-Met production.
| Groups | Initial pH | Fermentation Time (h) | Yeast Extract Concentration (g/L) | Inoculum Size (%) | L-Methionine Concentration (g/L) | Shaking Speed (rpm) | Temperature (°C) | 3-Met Concentration (g/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.5 | 36 | 0.2 | 0.4 | 3 | 180 | 28 | 2.28 |
| 2 | 4.75 | 44 | 0.4 | 0.6 | 4 | 195 | 30 | 3.13 |
| 3 | 5 | 52 | 0.6 | 0.8 | 5 | 210 | 32 | 3.73 |
| 4 | 5.25 | 60 | 0.8 | 1.0 | 6 | 225 | 34 | 3.62 |
| 5 | 5.5 | 68 | 1.0 | 1.2 | 7 | 240 | 36 | 2.66 |
2.4.4. Response Surface Analysis
Three components (fermentation time (A), yeast extract concentration (B), and initial pH (C)) that had the greatest influence were chosen for optimization utilizing the response surface analysis based on the significance order of factors impacting the yield of 3-Met from the Plackett–Burman design. The center point of the response surface analysis experiment was the highest point from the steepest ascent test. According to the principles of the Box–Behnken central composite design, a total of fifteen experiments with three factors and three levels, including three center point combinations, were designed using Design-Expert 11 software (Table 3).
Table 3.
Box–Behnken design and responses of the dependent variables.
| NO. | Fermentation Time (h) | Yeast Extract Concentration (g/L) | Initial pH | 3-Met Concentration (g/L) | |||
|---|---|---|---|---|---|---|---|
| A | Code A | B | Code B | C | Code C | Y | |
| 1 | 60 | 1 | 1.0 | 1 | 5.8 | 1 | 1.83 |
| 2 | 44 | −1 | 1.0 | 1 | 4.2 | −1 | 2.51 |
| 3 | 60 | 1 | 0.6 | 0 | 4.2 | −1 | 3.15 |
| 4 | 44 | −1 | 0.6 | 0 | 5 | 0 | 2.61 |
| 5 | 60 | 1 | 1.0 | 1 | 5 | 0 | 2.09 |
| 6 | 52 | 0 | 0.2 | −1 | 4.2 | −1 | 1.66 |
| 7 | 44 | −1 | 0.6 | 0 | 5.8 | 1 | 2.41 |
| 8 | 52 | 0 | 0.6 | 0 | 5 | 0 | 3.83 |
| 9 | 52 | 0 | 0.2 | −1 | 5.8 | 1 | 2.53 |
| 10 | 60 | 1 | 0.2 | −1 | 5 | 0 | 2.89 |
| 11 | 52 | 0 | 1.0 | 1 | 5.8 | 1 | 3.19 |
| 12 | 52 | 0 | 0.6 | 0 | 5 | 0 | 3.91 |
| 13 | 52 | 0 | 0.6 | 0 | 5 | 0 | 4.09 |
| 14 | 52 | 0 | 1.0 | 1 | 4.2 | −1 | 2.83 |
| 15 | 44 | −1 | 1.0 | 1 | 5 | 1 | 2.83 |
Note: the letter Y represents the 3-Met concentration.
2.5. Scale-Up Fermentation in Fermenter
The optimized medium composition and fermentation conditions were used for the scale-up fermentation experiment of S. fibuligera Y1402 in a 3 L fermenter. Considering the potential loss of 3-Met through exhaust emissions and the rapid growth of the yeast under high oxygen levels, the aeration rate of the fermenter was set at 0.5 air volume per culture volume per minute (vvm), the agitation speed was 300 rpm, the inoculum size was 0.8%, and the liquid volume was 1.8 L, taking cost into consideration. Other conditions were set according to the optimized conditions from the shake flask fermentation. Samples were taken every 8 h to measure the pH, cell density, and 3-Met concentration in the fermentation broth.
2.6. Analytical Methods
Yeast biomass was determined using the turbidity measurement (absorbance at 560 nm). The pH in the fermentation broth was measured using a pH meter. The analysis of volatile flavor compounds in the fermentation broth was performed using headspace solid-phase microextraction–gas chromatography–mass spectrometry, following the method described by Fan et al. [26]. The 3-Met concentration in the fermentation broth was determined using high-performance liquid chromatography, following the method described by Ma et al. [7].
2.7. Statistical Analysis
A one-way ANOVA (p < 0.05) with Tukey’s test was used to examine statistical differences in the evaluated techniques. Each experiment was performed in triplicate, and the experimental data were processed and plotted using Excel 2019 (Microsoft, Redmond, WA, USA), SPSS 24.0 (IBM Corp., New York, NY, USA), OriginPro 9.1 (OriginLab, Northampton, MA, USA), and Design-Expert 11 (Stat-Ease, Inc., Minneapolis, MN, USA).
3. Results and Discussion
3.1. Screening of High-Yield 3-Met Strains
A total of 94 strains were screened from high-temperature sesame-flavored Daqu using three different culture mediums, including 61 bacteria, 18 yeasts, and 15 molds. These strains were activated and cultured in the initial fermentation medium, and an analysis revealed that 35 strains were capable of producing 3-Met. This might be attributed to the specific sample of Daqu used, as previous studies have shown that 3-Met was an important flavor compound in sesame-flavored Baijiu [25]. Consequently, it is very possible that Daqu, which is utilized to promote fermentation and saccharification during the brewing of this particular variety of Baijiu, include 3-Met-producing strains. Among the tested strains (Table 4), most yeast strains were able to convert L-methionine into 3-Met, while most bacteria or molds were unable to do so. Among the strains capable of producing 3-Met, yeast generally exhibited higher concentrations compared with bacteria or molds, which was consistent with previous research [4,7]. This might be due to a higher enzymatic activity or expression levels of the enzymes involved in the Ehrlich pathway in yeast, which was responsible for the metabolism of L-methionine into 3-Met. Excitingly, 10 strains were found to produce 3-Met with a content exceeding 0.5 g/L, and all of these strains were yeast strains, which may also be related to the Daqu samples. Among them, yeast Y1402 produced the highest content of 3-Met, exceeding 2.0 g/L. Naturally, most strains that were able to produce 3-Met also resembled those from earlier studies in which the majority of microorganisms produced very little 3-Met [4,7]. To compare and analyze Y1402 with the previously isolated high-yield 3-Met yeast YHM-G within our team, we cultured these two yeasts under the same conditions in the initial fermentation medium. The results showed that both strains exhibited high 3-Met production capabilities, surpassing most of the reported high-yield 3-Met strains [7]. Furthermore, yeast Y1402 showed significantly higher 3-Met production compared with yeast YHM-G (Figure S1). Based on these results, we selected yeast Y1402 for further research.
Table 4.
Strains for 3-Met production in the present study.
| Strain Number | 3-Met Production (mg/L) | Strain Number | 3-Met Production (mg/L) | Strain Number | 3-Met Production (mg/L) | Strain Number | 3-Met Production (mg/L) |
|---|---|---|---|---|---|---|---|
| B1 | 0 ± 0 | B25 | 0 ± 0 | B49 | 24.57 ± 1.03 | Y12 | 217.73 ± 3.26 |
| B2 | 0 ± 0 | B26 | 0 ± 0 | B50 | 0 ± 0 | Y13 | 0 ± 0 |
| B3 | 0 ± 0 | B27 | 0 ± 0 | B51 | 0 ± 0 | Y14 | 895.29 ± 5.09 |
| B4 | 2.94 ± 0.22 | B28 | 0 ± 0 | B52 | 0 ± 0 | Y1402 | 2001 ± 10.37 |
| B5 | 0 ± 0 | B29 | 45.78 ± 2.12 | B53 | 0 ± 0 | Y15 | 0 ± 0 |
| B6 | 0 ± 0 | B30 | 0 ± 0 | B54 | 0 ± 0 | Y16 | 0 ± 0 |
| B7 | 53.85 ± 4.01 | B31 | 0 ± 0 | B55 | 0 ± 0 | Y17 | 1131 ± 9.27 |
| B8 | 5.52 ± 1.38 | B32 | 0 ± 0 | B56 | 0 ± 0 | M1 | 39.88 ± 2.03 |
| B9 | 30.57 ± 4.91 | B33 | 6.33 ± 0.24 | B57 | 34.65 ± 6.36 | M2 | 0 ± 0 |
| B10 | 0 ± 0 | B34 | 0 ± 0 | B58 | 0 ± 0 | M3 | 0 ± 0 |
| B11 | 0 ± 0 | B35 | 0 ± 0 | B59 | 0 ± 0 | M4 | 0 ± 0 |
| B12 | 0 ± 0 | B36 | 0 ± 0 | B60 | 52.49 ± 7.18 | M5 | 0 ± 0 |
| B13 | 0 ± 0 | B37 | 0 ± 0 | B61 | 39.09 ± 0.95 | M6 | 0 ± 0 |
| B14 | 0 ± 0 | B38 | 42.61 ± 2.00 | Y1 | 0 ± 0 | M7 | 0 ± 0 |
| B15 | 0 ± 0 | B39 | 0 ± 0 | Y2 | 1411.63 ± 17.39 | M8 | 0 ± 0 |
| B16 | 0 ± 0 | B40 | 0 ± 0 | Y3 | 1464.34 ± 8.46 | M9 | 0 ± 0 |
| B17 | 0 ± 0 | B41 | 41.20 ± 1.73 | Y4 | 1021.86 ± 5.19 | M10 | 43.63 ± 2.28 |
| B18 | 0 ± 0 | B42 | 0 ± 0 | Y5 | 1208.04 ± 17.30 | M11 | 54.58 ± 3.03 |
| B19 | 0 ± 0 | B43 | 0 ± 0 | Y6 | 1690.45 ± 14.22 | M12 | 30.39 ± 8.26 |
| B20 | 0 ± 0 | B44 | 0 ± 0 | Y7 | 0 ± 0 | M13 | 0 ± 0 |
| B21 | 52.73 ± 6.02 | B45 | 0 ± 0 | Y8 | 115.09 ± 2.97 | M14 | 34.83 ± 6.17 |
| B22 | 14.93 ± 3.17 | B46 | 0.73 ± 0.16 | Y9 | 559.45 ± 7.13 | M15 | 52.69 ± 2.04 |
| B23 | 49.32 ± 0.74 | B47 | 49.16 ± 3.36 | Y10 | 1488.61 ± 12.05 | ||
| B24 | 0 ± 0 | B48 | 0 ± 0 | Y11 | 0 ± 0 |
Note: The letter B represents bacteria; Y represents yeast; M represents mold.
3.2. Identification of Yeast Y1402
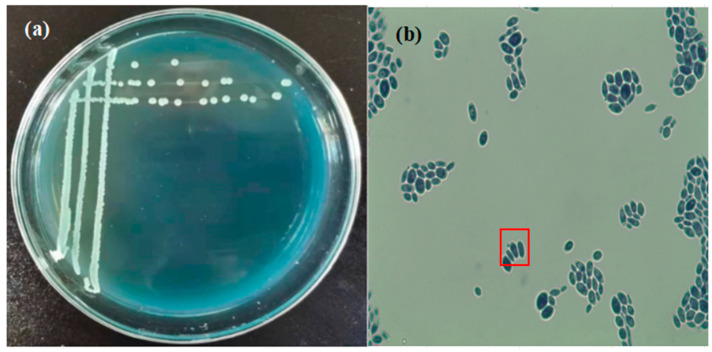
The colony morphology of strain Y1402 on WL medium was shown in Figure 1a. The colonies were white and small and round with a raised center. The color of the medium changed from blue-green to yellow in the area where the strain grew. The surface of the colonies was smooth, moist, and easy to pick. The cellular morphology of strain Y1402 was shown in Figure 1b, with most cells being oval-shaped and a few appearing elongated with budding at the top (indicated in the box in Figure 1b). These observations indicated that strain Y1402 exhibited the typical colony morphology and microscopic structure of yeast.
Figure 1.
Colony morphological characteristics of yeast Y1402 on the WL identification medium (a) and cell morphological characteristics under microscopy (10 × 100) (b). The asexual budding reproduction occurred at the ends of the cells was highlighted in the red square.
To determine the species of yeast Y1402, its physiological and biochemical characteristics were identified (Table S2). The results of the sugar fermentation test showed that yeast Y1402 utilized glucose to produce acid and gas. It also produced acid but not gas when utilizing D-galactose, D-maltose, and sucrose. However, it could not utilize L-rhamnose, D-xylose, D-arabinose, and lactose to produce acid or gas. In the carbon source assimilation test, yeast Y1402 used D-maltose, D-galactose, glucose, sucrose, ethanol, and D-ribose as the sole carbon sources for growth, and the growth was good. Although it could use inulin and glycerol as carbon sources for growth, the growth was slightly poorer compared with ethanol and D-ribose. It could not utilize D-trehalose, D-raffinose, mannitol, L-rhamnose, D-xylose, D-arabinose, lactose, and D-sorbitol as the sole carbon sources for growth. The nitrogen source assimilation test results showed that yeast Y1402 used urea, ammonium sulfate, L-lysine, and L-phenylalanine as the sole nitrogen sources for growth, and the growth was good. It could also use sodium nitrite and potassium nitrate as the sole nitrogen sources for growth, but the growth was poor. The indole test, methyl red test, citrate test, and starch hydrolysis test were positive, indicating that yeast Y1402 can secrete tryptophanase, utilize tryptophan to produce indole, ferment glucose to produce pyruvic acid or succinic acid, grow using sodium citrate as the sole carbon source, and produce amylase to hydrolyze starch. However, it showed negative results in the hydrogen sulfide test, Voges–Proskauer test, urea test, and gelatin liquefaction test. Based on these physiological characteristics being similar to S. fibuligera M33, it was preliminarily identified as S. fibuligera [27].
The obtained 26S rDNA D1/D2 sequence of yeast Y1402 was subjected to a BLAST comparison on NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 9 December 2023, accession number OR660109.1), and the results showed 100% similarity with S. fibuligera CBS:214.37 (accession number: MH867402.1), S. fibuligera LY41 (Accession number: KY705007.1), S. fibuligera KJJ81 (accession number: CP012816.1), and S. fibuligera ATCC36309 (accession number: CP015978.1). Combining morphological observations, physiological and biochemical characteristics, and molecular biology, yeast Y1402 was identified as S. fibuligera. S. fibuligera is common non-brewing yeast that plays an important role in fermented foods. It can produce enzymes such as lipase, protease, and amylase to promote the hydrolysis of nutrients in raw materials and produce flavor compounds such as ethyl acetate, phenethyl alcohol, isoamyl acetate, and phenethyl acetate [28,29,30,31]. It is important flavor-producing yeast in the fermentation process of food production.
3.3. Determination of S. fibuligera Y1402 Performance
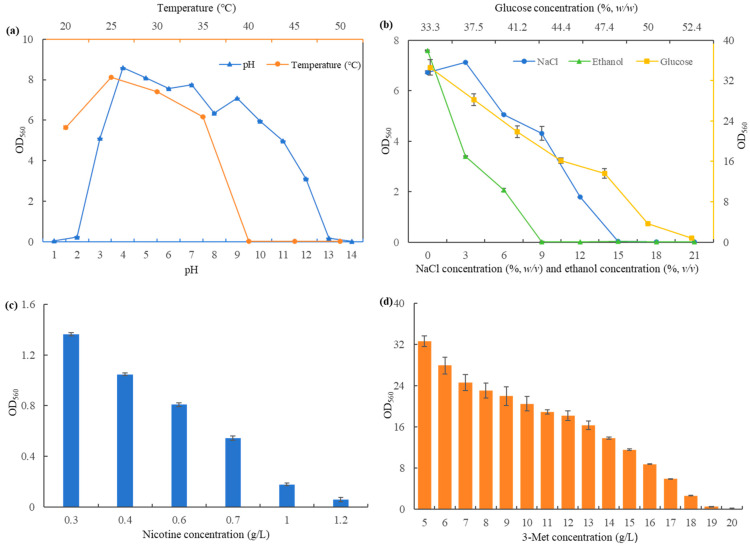
The growth temperature, pH, and tolerance of S. fibuligera Y1402 to sugar, NaCl, ethanol, nicotine, and 3-Met is shown in Figure 2. The optimal growth temperature for S. fibuligera Y1402 was 25 °C, with a maximum tolerance of 40 °C (Figure 2a), which was consistent with most yeast and indicated its suitability for fermentation in products like Baijiu [32]. S. fibuligera Y1402 exhibited optimal growth at a pH of 4 and tolerated a wide range of pH values from 2 to 13 (Figure 2a), making it adaptable to various fermentation environments, such as the production of Baijiu and Huangjiu. Its lowest pH tolerance was similar to other yeast strains known for acid tolerance [33,34]. S. fibuligera Y1402 also demonstrated high tolerance to sugar, being able to grow even in the presence of 50% glucose concentration (Figure 2b), surpassing most yeast strains [7,26,35]. Regarding NaCl concentration, the yeast density of S. fibuligera Y1402 initially increased and then decreased as the NaCl concentration raised (Figure 2b). At a NaCl concentration of 3%, S. fibuligera Y1402 growth was promoted, indicating that its growth was favored under certain suitable NaCl conditions. However, when the concentration exceeded 3%, the high osmotic pressure inhibited the growth of S. fibuligera Y1402. Its maximum NaCl tolerance was as high as 15%, which was consistent with the NaCl tolerance of Clavispora lusitaniae YX3307 and higher than that of Pichia kudriavzevii YF1702, but lower than that of H. burtonii YHM-G [7,26,35]. In terms of ethanol tolerance, S. fibuligera Y1402 showed a decrease in cell density as ethanol concentration increased, and it hardly grew when the ethanol concentration exceeded 6% (Figure 2b). This indicated that S. fibuligera Y1402 was sensitive to ethanol, similar to C. lusitaniae YX3307 but lower than H. burtonii YHM-G [7,26]. Fortunately, the ethanol concentration in the production process of Baijiu was generally between 2 and 4%, suggesting that this yeast could be applied in Baijiu production [7,26,36]. To explore the potential application of S. fibuligera Y1402 in tobacco products, its nicotine tolerance was analyzed. The results showed that as the nicotine concentration increased, the inhibition of S. fibuligera Y1402 growth intensified. The maximum nicotine tolerance of S. fibuligera Y1402 was 1.2 g/L (Figure 2c), which was lower than the nicotine content in tobacco leaves (usually 1.5–3.5%) [37]. However, our research had revealed that S. fibuligera Y1402 could grow on tobacco leaves, possibly due to the low local nicotine content on the leaves and the influence of the structure of the tobacco leaves, which reduced the damage to yeast cells. To investigate its ability to produce 3-Met, the tolerance of S. fibuligera Y1402 to 3-Met was analyzed. As observed in Figure 2d, the growth of S. fibuligera Y1402 was increasingly inhibited with higher concentrations of 3-Met. The maximum tolerance to 3-Met was found to be 18 g/L, which was much lower than that of H. burtonii YHM-G and S. cerevisiae CEN.PK113-7D [5,7]. However, subsequent experimental results revealed that high tolerance to 3-Met only indicated the potential for the high yield of 3-Met but did not necessarily mean that higher tolerance would lead to a higher yield of 3-Met. Considering the above characteristics, S. fibuligera Y1402 has the potential for application in fermentation products such as Baijiu and tobacco.
Figure 2.
The range of growth temperature and pH (a); tolerance of glucose, NaCl, and ethanol (b); nicotine (c); and 3-Met (d) of strain Y1402.
3.4. Optimization of S. fibuligera Y1402 Fermentation Conditions for 3-Met Production
3.4.1. Single-Factor Experiment
Although the synthesis of 3-Met occurs through the Ehrlich pathway, glucose plays a significant role in providing energy for microbial growth and metabolic activities. It serves as a carbon skeleton that is an essential substance in microbial activities [26,38]. Therefore, the concentration of glucose has a certain influence on the synthesis of 3-Met by yeast. Increasing the glucose concentration at lower glucose concentrations facilitated the development and metabolism of S. fibuligera Y1402, which in turn facilitated the synthesis of 3-Met, as Supplemental Figure S2a illustrates. But when the concentration of glucose raised, S. fibuligera Y1402 grew quickly, which resulted in insufficient oxygen being supplied to the fermentation system. This raised the production of other higher alcohols that were excessive and might be hazardous to S. fibuligera Y1402, which in turn caused a reduction in the concentration of 3-Met generated. Additionally, it altered the metabolic route of S. fibuligera Y1402 in converting L-methionine to 3-Met [7,11,39]. From the experimental results (Figure S2a), the maximum concentration of 3-Met was recorded in S. fibuligera Y1402 when the glucose content was between 40 and 55 g/L. This was more than the ideal glucose concentration for S. cerevisiae SC408 to produce 3-Met, but it was comparable with the glucose concentration needed for H. burtonii YHM-G to produce 3-Met [7,11]. This difference might be attributed not only to differences in the initial fermentation medium but also to the different yeast species.
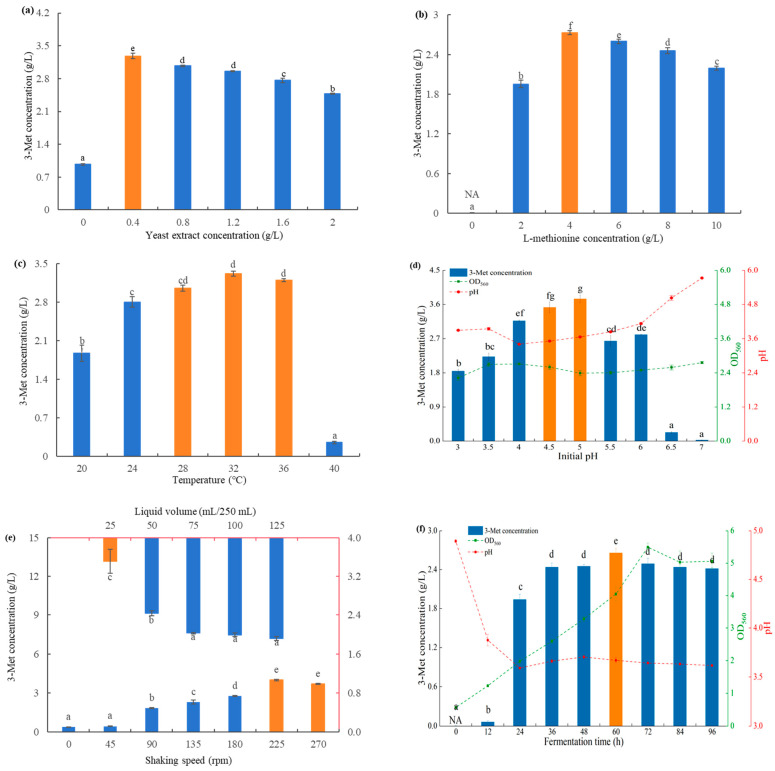
The Ehrlich pathway is an important pathway for the microbial synthesis of higher alcohols and other compounds. However, the nitrogen supply in the fermentation medium may readily affect this process. Specifically speaking on the synthesis of 3-Met in the present investigation, the Ehrlich metabolism route in yeast would be somewhat inhibited by the presence of nitrogen sources other than L-methionine, leading to a reduction in 3-Met synthesis [1,38]. However, research has shown that adding a certain quantity of yeast extract in the fermentation medium may encourage yeast to produce 3-Met [7]. This might be because yeast extract not only serves as an organic nitrogen source for microbial growth but also provides important growth factors such as amino acids, vitamins, and biotin for microbial growth and reproduction [3,7]. When yeast extract was present, the amount of 3-Met synthesized by S. fibuligera Y1402 was higher compared with when yeast extract was absent, as shown in Figure 3a. Moreover, a concentration of 0.4 g/L of yeast extract produced the maximum quantity of 3-Met. However, the quantity of 3-Met produced by S. fibuligera Y1402 decreased when the yeast extract concentration was raised over this threshold. This finding initially showed that S. fibuligera Y1402 benefited from the presence of yeast extract in the synthesis of 3-Met. Additionally, it showed that yeast extract functioned as both an Ehrlich pathway inhibitor and a growth promoter for yeast. When yeast extract was present in greater quantities, it blocked the Ehrlich pathway and functioned as a nitrogen source; however, at lower concentrations, it promoted yeast growth exclusively as a growth factor. The yeast extract concentration required to achieve optimum 3-Met synthesis by S. fibuligera Y1402 was comparable with that of K. lactis KL71 but lower than that of H. burtonii YHM-G, S. cerevisiae EC-1118, and S. cerevisiae SC408 [7,11,13,40]. This difference is mainly attributed to the metabolic variations among different yeast species.
Figure 3.
Effect of yeast extract concentration (0, 0.4, 0.8, 1.2, 1.6, and 2.0 g/L) (a), L-methionine concentration (0, 2, 4, 6, 8, and 10 g/L) (b), temperature (20, 24, 28, 32, 36, and 40 ℃) (c), initial pH (3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0) (d), shaking speed ((0, 45, 90, 135, 180, 225 and 270 rpm) and liquid volume (25, 50, 75, 100, and 125 mL/250 mL) (e), and fermentation time (0, 12, 24, 36, 48, 60, 72, 84, and 96 h) (f) on 3-Met concentration. The same letters in the column indicate that the data do not differ significantly at 5% probability using Tukey’s test. The orange bar means the best conditions on 3-Met concentration. Note: the letter NA represents no detection of 3-Met.
Surfactants are a type of amphiphilic chemicals that can interact with microbial cell membranes to change their permeability through bilayer interactions. This influences the production of intracellular chemicals by microbes as well as their intake of nutrients. Furthermore, surfactants have the ability to control the amount of microbial enzymes secreted and affect the activity of these enzymes [41,42]. As a result, surfactants significantly affect the way that microbial cells use energy. Figure S2b demonstrates that the other surfactant types had no discernible influence on the synthesis of 3-Met by S. fibuligera Y1402 with the exception of Tween 80 and Tween 60, which had a small boosting effect. The effect of surfactants on the formation of 3-Met by H. burtonii YHM-G, on the other hand, was different and might have resulted from small differences in the yeast cell’s membrane composition or the activity of associated Ehrlich pathway enzymes [8]. Moreover, Tween 80 did not significantly alter its action at different doses, even though it promoted the synthesis of 3-Met by S. fibuligera Y1402 in the range of 2–6.4 g/L (as shown in Figure S2c). This indicated that Tween 80 had good fusion properties with the S. fibuligera Y1402 cell membrane, even at higher concentrations, without causing membrane disruption, electrolyte leakage, or metabolic imbalance, as described in other studies [43]. This was similar to the effect of Tween 80 on the synthesis of 3-Met by H. burtonii YHM-G [7]. Considering cost, a concentration of 2 g/L of Tween 80 was chosen, and no further optimization of its concentration was performed in subsequent experiments.
Through the Ehrlich pathway, 3-Met might be produced from L-methionine [8,44]. In summary, by undergoing transamination, decarboxylation, and reduction processes, 3-Met was produced from L-methionine [5]. Due to the fact that yeast synthesizes 3-Met through the Ehrlich pathway, the importance of L-methionine concentration in yeast for the synthesis of 3-Met is self-evident [13]. S. fibuligera Y1402 was shown to be unable to synthesize 3-Met in the absence of L-methionine, which was in accordance with other reports, as seen in Figure 3b [1,7]. Yeast was known to manufacture 3-Met via the Ehrlich pathway and was unable to use carbohydrates for de novo synthesis. The results also showed that the synthesis of 3-Met by S. fibuligera Y1402 was significantly impacted by varying amounts of L-methionine. Through the Ehrlich pathway, the rate and yield of 3-Met synthesis were constrained at low doses of L-methionine. However, at high L-methionine concentrations, yeast generated more 3-Met and accumulated more 3-methylthiopropionic acid, which was hazardous to the yeast itself [5]. The yeast changed regular metabolic pathways within the yeast cells by desulfurizing L-methionine to lower its effective concentration and prevent self-harm and excessive formation of 3-methylthiopropionic acid [3,13,45]. This ultimately resulted in a reduction in the capacity to synthesize 3-Met. The findings indicated that 4 g/L of L-methionine was the ideal concentration for S. fibuligera Y1402 to synthesize 3-Met. These findings were in line with those obtained for H. burtonii YHM-G and the majority of strains that have been published [3,7,13,46].
The influence of L-methionine on the synthesis of 3-Met by yeast was not only reflected in its concentration effect but also in the timing of its addition [7]. In contrast to H. burtonii YHM-G, the concentration of 3-Met synthesized by S. fibuligera Y1402 steadily declined with the delayed addition of L-methionine, as seen in Supplemental Figure S1d [7]. There are two possible causes for this: (1) L-methionine was added to the medium early in fermentation, which helped the yeast grow; (2) S. fibuligera Y1402 had a short lag phase, good environmental adaptability, and the ability to grow quickly in the medium under the conditions present. It is possible that this amino acid is necessary for the growth of S. fibuligera Y1402. Early in the fermentation process, S. fibuligera Y1402 may swiftly adapt to the Ehrlich pathway, supplying the energy or electrochemical gradient required for the production of 3-Met [7,13]. For these reasons, introducing L-methionine during the early stages of fermentation not only encouraged the development of S. fibuligera Y1402, but also gave the Ehrlich pathway enough time and circumstances to convert L-methionine into 3-Met. The differing needs for yeast extract concentration between S. fibuligera Y1402 and H. burtonii YHM-G may provide an explanation for this conjecture [7]. The concentration of yeast extract in the current medium was adequate to fulfill S. fibuligera Y1402’s demands for the synthesis of 3-Met, allowing for the organism’s fast growth and provision of the energy or electrochemical gradient required for the synthesis of 3-Met. But insufficient yeast extract in the first fermentation medium prevented H. burtonii YHM-G from growing, and this organism lacks the energy and electrochemical gradient needed to convert L-methionine into 3-Met. Therefore, this yeast may be able to synthesize 3-Met if L-methionine is added later [7]. This difference was mainly due to the inherent differences in the characteristics of the two yeasts.
Temperature is a crucial parameter that significantly affects microbial growth. When the temperature is too low, it decreases the fluidity of microbial cell membranes and the enzymatic activity, resulting in slow growth or even inhibition of microbial growth; conversely, when the temperature is too high, it can lead to the destruction of microbial cell structure and the inactivation of enzymes, causing microbial death [47]. Therefore, only suitable temperatures are favorable for the growth and metabolism of microorganisms, allowing them to accumulate more target products. S. fibuligera Y1402 was capable of producing 3-Met over a wide range of temperatures, with the most favorable temperature range for its synthesis being between 28 and 36 °C, as shown in Figure 3c. This was consistent with the optimal growth temperature range of S. fibuligera Y1402 and with the reported optimal temperature range for yeast synthesis of 3-Met in most studies [1,7].
pH is another important parameter that regulates microbial growth. It affects microbial growth and metabolic activities by altering the ion state of nutrients in the medium, the membrane potential of microbial cell membranes, the membrane permeability, and the functional activity of membrane proteins [26,40]. Through analysis, it was found that the initial pH had a significant impact on the yield of 3-Met by S. fibuligera Y1402 (Figure 3d). At an initial pH of 3-3.5, the yield of 3-Met was low, below 2.5 g/L. As the initial pH increased, the concentration of 3-Met produced by S. fibuligera Y1402 steadily increased. At an initial pH of 4.5-5, S. fibuligera Y1402 produced the highest amount of 3-Met, exceeding 3.2 g/L. Subsequently, the yield of 3-Met decreased with increasing pH, and at pH above 6.5, the yield of 3-Met was extremely low. This was similar to H. burtonii YHM-G, which also originated from the Baijiu fermentation environment [7]. It was possible that both yeasts had undergone long-term adaptation to the slightly acidic environment of Baijiu fermentation, resulting in the optimal pH of the enzymes involved in the Ehrlich pathway in these two yeasts being slightly acidic [1,7,13]. This can be inferred from the results of pH and biomass after fermentation under different conditions.
The inoculum size affects the rate of microbial growth and duration of fermentation cycles and has an important impact on the accumulation of target products and production costs [48]. Although the highest content of 3-Met produced by S. fibuligera Y1402 was observed at an inoculum size of 0.8%, overall, it was capable of a high yield of 3-Met within the range of a 0.1% to 3.2% inoculum size, as shown in Figure S1e. This indicated that S. fibuligera Y1402 had good environmental adaptability or that the initial fermentation medium’s nutrient composition and content were suitable for the growth of S. fibuligera Y1402. However, when the inoculum size reached 6.4%, the content of 3-Met produced by S. fibuligera Y1402 decreased. This might be due to the yeast growing and reproducing too quickly, consuming more nutrients, and causing a severe lack of oxygen in the fermentation system. As a result, the strain’s ability to synthesize 3-Met was compromised. This was similar to the fermentation of 3-Met by H. burtonii YHM-G, which also required a similar inoculum size [7].
Based on previous research, it was known that yeast metabolizes L-methionine into 3-Met through the Ehrlich pathway, which is an aerobic process. Under conditions where the oxygen content is suitable, it may promote the expression of enzymes involved in the Ehrlich pathway, leading to the production of higher concentrations of 3-Met, even up to 10 times higher than under conditions of insufficient oxygen [1,7,13]. In the shake flask fermentation, the shaking speed and liquid volume were two important parameters that affected the oxygen content. The 3-Met concentration synthesized by S. fibuligera Y1402 at a speed of 45 rpm, as shown in Figure 3e, exhibited no significant variation when compared with static fermentation. However, this concentration was much greater under other speed parameters than under static fermentation. Additionally, the synthesis of 3-Met increased with increasing shaking speed, reaching a maximum of 3.9 g/L at a shaking speed of 225 rpm, which was approximately 10 times higher than under static conditions. This indicated that the yeast benefited from higher oxygen content for the synthesis of 3-Met. This was further confirmed by optimizing the liquid volume condition, as the highest concentration of 3-Met was achieved at lower liquid volumes. However, as the speed continued to increase, the yield of 3-Met by S. fibuligera Y1402 showed a slight decreasing trend. This was related to the dual effect of strong shear forces generated at high speeds causing mechanical damage to yeast cells and the higher production of 3-methylthiopropionic acid under high oxygen content causing chemical harm to yeast cell [6,7]. From the perspective of speed, the optimal speed for 3-Met production by S. fibuligera Y1402 was similar to that of S. cerevisiae S288C-CYS3 and higher than the optimal speed required by H. burtonii YHM-G [6,7]. From the perspective of liquid volume, the optimal liquid volume for S. fibuligera Y1402 was lower than that of H. burtonii YHM-G, indicating that S. fibuligera Y1402 required higher oxygen content for 3-Met production compared with H. burtonii YHM-G [7]. This might be due to the differences in their ability to resist physical shear forces and chemical toxicity, as well as the differences in the oxygen requirements of metabolism-related enzymes.
The optimal fermentation time is crucial for maximizing the profitability of industrial production using microorganisms, aiming to achieve the maximum accumulation of target products in the shortest time possible. The optimal fermentation time varies depending on the microbial species, target metabolites, fermentation medium, and cultivation conditions and requires targeted exploration. As shown in Figure 3f, S. fibuligera Y1402 was able to start converting L-methionine into 3-Met at the initial stage of fermentation (12 h). When the fermentation time reached 24 h, the concentration of 3-Met reached over 70% of the maximum value. Compared with H. burtonii YHM-G, this yeast had the characteristic of rapidly converting L-methionine into 3-Met, which might explain why S. fibuligera Y1402 started adding L-methionine earlier than H. burtonii YHM-G [7]. This can also be confirmed by the pH and cell density change trends of the two strains. However, compared with the optimal fermentation time of 48 h for H. burtonii YHM-G, S. fibuligera Y1402 took a slightly longer time, 60 h, to reach the highest concentration of 3-Met [7]. This time was much shorter than the fermentation time required by S. cerevisiae SC408, which could be attributed to the differences in microbial strains as well as variations in medium composition and fermentation conditions [11].
3.4.2. Plackett–Burman Design
Based on the results of single-factor experiments, it was determined that the optimal surfactant for S. fibuligera Y1402 was Tween 80, with an addition amount of 2 g/L. The optimal timing for adding L-methionine was 0 h. The impact of several other factors on the production of 3-Met was also analyzed. Without considering the interaction effects, the optimal levels of these factors were determined. However, in reality, there were interactions between many factors, so further optimization of these factors was needed. To simplify the subsequent optimization process, the factors were reduced by using a Plackett–Burman design. The factors that significantly affect the production of 3-Met by S. fibuligera Y1402 were selected, and a sequence of the effects of these variables was established. These factors include glucose concentration, yeast extract concentration, L-methionine concentration, temperature, initial pH, inoculum size, shanking speed, liquid volume, and fermentation time. Table 1 displays the results. The table shows that the range of the yield of 3-Met generated by S. fibuligera Y1402 was 0.50 g/L to 3.83 g/L. The yeast yielded the largest amount of 3-Met in the tenth group when all factors—aside from yeast extract concentration—were at the ideal values found in the single-factor studies. This suggested that the interaction effects between the factors may not be significant. It is worth noting that although there was no significant difference between the maximum 3-Met production in the Plackett–Burman design experiment and the highest production in the single-factor experiment result, the two fermentation conditions for the highest 3-Met production were different. There was a lower concentration of yeast extract in the Plackett–Burman design experiment compared with in the single-factor experiment, which reduced the cost of 3-Met production.
Using the acquired findings, a multiple linear regression equation was fitted and encoded. The resulting equation is as follows:
| Y = 2.44 + 0.0291 A + 0.2849 B + 0.2421 C + 0.2191 D + 0.3153 E+ 0.2992 F+ 0.2528 G − 0.0574 H + 0.2224 I |
The variance analysis of the results yielded a p value of 0.0134 (p < 0.05), indicating that the model was significant. The coefficient of determination (R2) for the regression equation was 0.9658, further demonstrating the reliability of the model and its ability to predict the results well. From the regression equation coefficients (Table 1), it was inferred that the liquid volume had a negative effect on the synthesis of 3-Met by yeast, while the other eight factors had positive effects. Through the analysis of the significance of each factor, it was evident that initial pH, fermentation time, yeast extract concentration, inoculum size, L-methionine concentration, shaking speed, and temperature had significant effects on the synthesis of 3-Met by yeast. The significance order of these factors followed the current writing order, indicating the need for further optimization research. On the other hand, the liquid volume and glucose concentration did not have a significant impact, so these two factors should be set at their optimal levels as determined by the single-factor experiment.
3.4.3. Steepest Ascent Test
Combining the results of the single-factor experiments, Plackett–Burman design, practical experience, and principles of the steepest ascent test, the direction and step size of each factor in the steepest ascent test were determined. Results obtained based on this design are shown in Table 2. From these results, it was observed that the concentration of 3-Met synthesized by S. fibuligera Y1402 initially increased and then decreased with the increase in the experimental group. Under the conditions of the third experimental group, i.e., initial pH 5, fermentation time 52 h, yeast extract concentration 0.6 g/L, inoculum size 0.8%, L-methionine concentration 5 g/L, shaking speed 210 rpm, and temperature 32 °C, the highest yield of 3-Met synthesized by S. fibuligera Y1402 was 3.73 g/L. It was worth noting that this highest level was lower than the highest level obtained in the Plackett–Burman design, which might be due to the suboptimal step size design for each factor. However, as the difference between the two was not significant, it was decided to conduct subsequent response surface analysis using the factor levels corresponding to the third experimental group in the steepest ascent test.
3.4.4. Response Surface Analysis
Based on the analysis of the Plackett–Burman design results, the significance of various factors affecting the production of 3-Met by S. fibuligera Y1402 was determined. Fermentation time, yeast extract concentration and initial pH were the three parameters that were chosen. The levels of the three factors were set as the third group in the steepest ascent test. According to the Box–Behnken experimental design principle, a total of 15 experiments with 3 factors and 3 levels were conducted, as shown in Table 3. The yield of 3-Met ranged from 1.66 to 4.09 g/L. The software Design-Expert 11.0 was used to perform a multivariate regression analysis and fit a multivariate quadratic equation regression to the above results. The regression coded equation obtained is as follows:
| Y = 3.95 + 0.5339A + 0.4763B+ 0.2444C − 0.8623A2 − 1.13B2 − 0.2833C2 − 0.9223AB + 0.0206 AC − 0.1504BC |
From the analysis of variance, it was concluded that the pattern was p < 0.05, indicating that the model was significant. The R2 of the quadratic regression equation was 0.9891, indicating that the equation fitted the experiment well with small errors. The insignificance of the lack-of-fit term implied a good correlation between the actual and predicted values. The yield of 3-Met was reflected and predicted by this model. The variation of the dependent variable depends on the changes in the independent variables, and the impact of the independent variables on the dependent variable can be reflected through the significance in the analysis of variance. In the regression equation, the p values of the first-order terms A (fermentation time), B (yeast extract concentration), and C (initial pH) were all less than 0.05, indicating that these three factors had a significant linear relationship with the synthesis of 3-Met by S. fibuligera Y1402 (Table 5). The order of influence of the three factors on the yield of 3-Met was inconsistent with the results of the Plackett–Burman tests, with B > C > A. This was related to differences in principles, i.e., the number and levels of factors involved in the two designs. The interaction terms AC and BC were significant, while AB was not significant. In the quadratic terms, the terms A2, B2, and C2 were all significant (p < 0.05), indicating significant curved effects between the fermentation time, yeast extract concentration, initial pH, and yield of 3-Met.
Table 5.
Regression coefficients and their significances for 3-Met production from the results of the Box–Behnken design.
| Source | Sum of Squares | DF | Mean Square | F Values | p Values | Significance |
|---|---|---|---|---|---|---|
| Model | 7.22573 | 9 | 0.80286 | 49.06 | 0.000 | *** |
| A—Fermentation time | 1.23168 | 1 | 1.23168 | 75.26 | 0.000 | *** |
| B—Yeast extract concentration | 1.27371 | 1 | 1.27371 | 77.83 | 0.000 | *** |
| C—Initial pH | 0.37036 | 1 | 0.37036 | 22.63 | 0.005 | ** |
| A2 | 0.22626 | 1 | 0.22626 | 13.83 | 0.014 | * |
| B2 | 2.41522 | 1 | 2.41522 | 147.58 | 0.000 | *** |
| C2 | 2.21996 | 1 | 2.21996 | 135.65 | 0.000 | *** |
| AB | 0.00101 | 1 | 0.00101 | 0.06 | 0.814 | - |
| AC | 0.10892 | 1 | 0.10892 | 6.66 | 0.049 | * |
| BC | 1.92710 | 1 | 1.92710 | 117.75 | 0.000 | *** |
| Residual | 0.08183 | 5 | 0.01637 | |||
| Lack of Fit | 0.04553 | 3 | 0.01518 | 0.84 | 0.585 | - |
| Pure Error | 0.03630 | 2 | 0.01815 | |||
| Cor Total | 7.30756 | 14 | ||||
| R2 = 0.9891 | R2Adj = 0.9694 |
Note: “-”, not significant (p > 0.05); “*”, significant at the 5% level (p < 0.05); “**”, significant at the 1% level (p < 0.01); “***”, significant at the 0.1% level (p < 0.001).
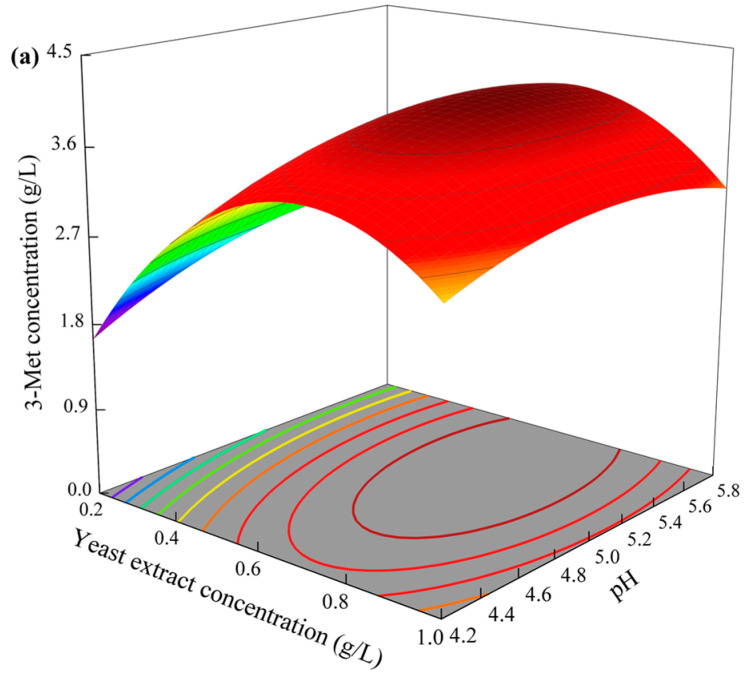
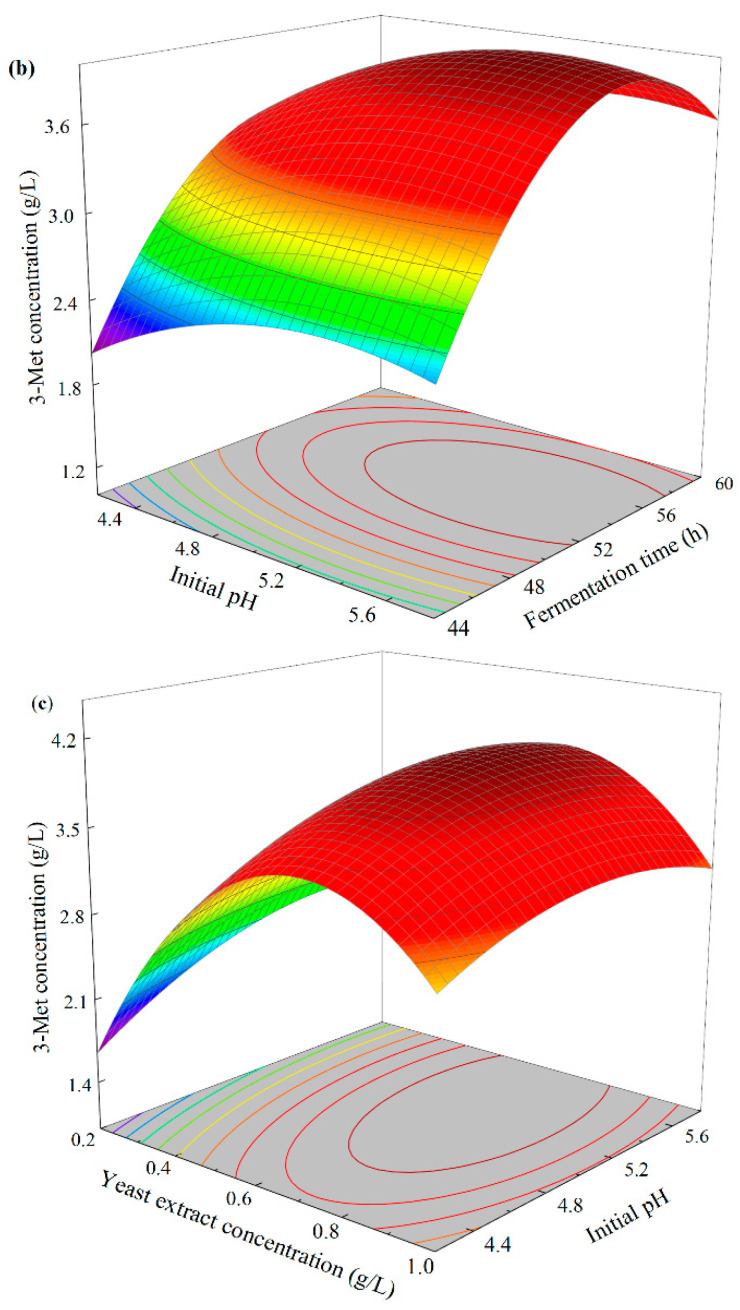
Based on the regression equation, a three-dimensional graph of the pairwise interaction effects between the three factors was plotted. Figure 4a indicates that the yield of 3-Met showed a clear increasing followed by a decreasing trend with increasing yeast extract concentration or fermentation time. The yield of 3-Met synthesized by S. fibuligera Y1402 increased initially and then decreased with increasing initial pH, but the overall trend was not significant, as shown in Figure 4b. However, with fermentation time, there was a significant increase followed by a decrease. According to Figure 4c, the yield of 3-Met also showed an initial increase followed by a decrease with increasing initial pH, but the impact was also not significant. However, with increasing yeast extract concentration, there was a clear increase followed by a decrease.
Figure 4.
The three-dimensional graph of variables (fermentation time, yeast extract concentration, and initial pH) on the 3-Met concentration response using the Box–Behnken design. (a) Fermentation time and yeast extract concentration; (b) fermentation time and initial pH; (c) yeast extract concentration and initial pH.
The regression equation was then used to determine the optimal conditions for maximizing the 3-Met yield by setting the partial derivatives of the equation to zero with respect to the independent variables. From the regression equation, it was concluded that the highest yield of 3-Met produced by S. fibuligera Y1402 occurred at an initial pH of 5.3, fermentation time of 54 h, and yeast extract concentration of 0.63 g/L, with a predicted value of 4.10 g/L. To validate this, three repeated experiments were conducted under the same conditions, and the yield of 3-Met produced by the yeast was found to be 4.03 g/L, which was very close to the predicted value with a deviation of only 1.7%, proving the reliability of the model. Compared with the reported studies (Table 6), the yield of 3-Met produced by S. fibuligera Y1402 was less only than that produced by the genetically modified strain S. cerevisiae S288C-AR010 and higher than that produced by all reported natural strains, making it the highest natural strain to produce 3-Met currently reported [18].
Table 6.
Summary of 3-Met production by strains with a content exceeding 0.5 g/L.
| Strain Classify | Strain | Culture Institution | L-Met Concentration (g/L) | 3-Met Production (mg/L) | Reference |
|---|---|---|---|---|---|
| Natural strain |
S. cerevisiae S288C |
Beijing Technology and Business University, Beijing 100048, China | 10 | 590 | [14] |
|
S. cerevisiae SC408 |
Beijing Technology and Business University, Beijing 100048, China | 4 | 1600 | [11] | |
| H. burtonii YHM-G | Beijing Technology and Business University, Beijing 100048, China | 6 | 3160 | [7] | |
| K. lactis KL71 | National University of Singapore, Singapore 117543 | - | 990 | [13] | |
| S. cerevisiae SC57 | Beijing Technology and Business University (BTBU), Beijing 100048, China | 4 | 1600 (fed-batch fermentation without D101); 2260 (fed-batch fermentation with D101) | [12] | |
| S. fibuligera Y1402 | Beijing Technology and Business University (BTBU), Beijing 100048, China | 5 | 4030 | This study | |
| Engineered strain |
S. cerevisiae S288C-CYS3 |
Beijing Technology and Business University, Beijing 100048, China | 4 | 690 | [6] |
|
S. cerevisiae s288c-ARO10 |
Beijing Technology and Business University, Beijing 100048, China | 10 | 900 | [16] | |
| S. cerevisiae C3 | Beijing Technology and Business University, Beijing 100048, China | 10 | 600 | [49] | |
| S. cerevisiae S288C | Beijing Technology and Business University, Beijing 100048, China | 10 | 940 | [49] | |
|
S. cerevisiae AR8 |
Beijing Technology and Business University, Beijing 100048, China | 10 | 760 | [14] | |
| S. cerevisiae S288C-AR010 | Beijing Technology and Business University, Beijing 100048, China | 5 | 4380 | [18] | |
| S. cerevisiae CEN.PK113-7D | Biochemical Engineering Group, Theodor-Heuss-Allee 25, 60486 Frankfurt am Main, Germany |
2200 | [5] | ||
| S. cerevisiae (co-expression of ARO8 and ARO100 | School of Food and Chemical Engineering, Beijing Technology and Business University, Beijing 100048, China | 10 | 3240 | [17] |
3.5. Scale-Up Fermentation in a 3 L Fermenter
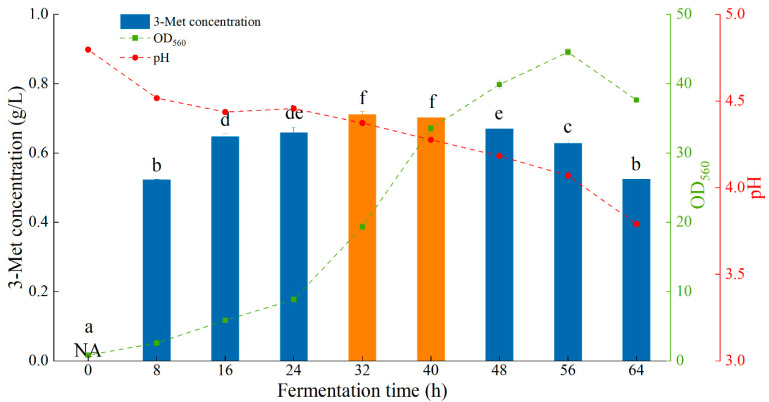
Based on the above optimized conditions, the production of 3-Met by Y1402 in a 3 L fermenter was investigated. As the fermentation duration was extended, as seen in Figure 5, the concentration of 3-Met generated by Y1402 rose and peaked at 32–40 h. It produced the maximum yield of 3-Met at this point in the logarithmic growth phase, which was earlier than the phase for this strain and H. burtonii YHM-G in the shaking flask. This might be because of the strain’s characteristics and the varied oxygen supply [7]. The difference in the two yeasts’ results supported the earlier theory about optimizing the moment at which L-methionine is added. The yield of 3-Met decreased as fermentation continued. This could be because the cell used the 3-Met as a source of nutrition and transformed it into other substances because there were not enough nutrients available, or it could be because the cell died and prevented the 3-Met from being synthesized further, causing the 3-Met that had already been produced to be lost in the exhaust gas. Unfortunately, the yield of 3-Met was not ideal through scale-up culture, mainly due to the large amount of 3-Met lost in the exhaust gas, similar to the ethyl acetate produced reported in our previous study (Supplementary Figure S3). In the future, other types of fermenters, such as centrifugal fermenters, will be used to explore the scaled-up cultivation with the hope of improving the 3-Met yield of Y1402.
Figure 5.
The result of 3-Met production by yeast Y1402 in a 3 L fermenter. The same letters in the column indicate that the data do not differ significantly at 5% probability using Tukey’s test. The orange bar means the best conditions on 3-Met concentration. Note: the letter NA represents no detection of 3-Met.
3.6. Production of Aromatic Compounds
S. fibuligera Y1402 produced 28 kinds of flavor substances in SHM, including 8 alcohols, 3 esters, 2 aldehydes, 6 phenols, 2 acids, 3 furanones, 1 azole, and 3 other flavor substances (Table 7). A comparative analysis with the original flavor substances in the SHM revealed that only four flavor substances, phenethyl alcohol, (E)-9-octadecenoic acid ethyl ester, dimethyl phthalate, and 2,4-dimethyl-benzaldehyde, were present before and after fermentation. A total of 5 flavor substances were found only in the SHM, while 24 flavor substances were present in the post-fermentation medium. This indicated that S. fibuligera Y1402 converted the original flavor substances in the medium using specific metabolic pathways as well as produced new flavor substances. In addition, among the four common flavor substances, phenethyl alcohol and 2,4-dimethyl-benzaldehyde in the post-fermentation medium were derived not only from the original components of medium but also from the utilization of nutrients by S. fibuligera Y1402 [35]. Through analysis, it was found that the flavor substances produced by this yeast were mainly composed of compounds with floral, sweet, creamy, roasted nuts, and clove-like aromas. Representative flavor substances included (Z)-3,7-dimethyl-3,6-octadien-1-ol,2-methyl-1-propanol, (R)-2-octanol, 3-methyl-1-butanol, phenylethyl alcohol, 4-ethyl-2-methoxy-phenol, 2-methoxy-4-vinylphenol, and 2,3-dihydro-benzofuran. These flavor substances were in line with the current trend of a light and sweet style in Baijiu development, as well as the roasted aroma characteristic of sesame-flavored Baijiu, which may be related to the yeast originating from the environment of sesame-flavored Baijiu brewing [50,51]. It was worth noting that, consistent with the aforementioned optimization results, S. fibuligera Y1402 cannot synthesize 3-Met through a de novo synthesis pathway when there was no or very little L-methionine available. This once again confirmed that S. fibuligera Y1402 produced 3-Met through the Ehrlich pathway [7]. Therefore, in fermentation products that required the synthesis of 3-Met by S. fibuligera Y1402, a certain amount of L-methionine needed to be present in the fermentation product matrix or achieved by constructing a workable synthetic microbial community [19].
Table 7.
The volatile compounds in SHM with or without Y1402 (µg/L).
| Volatile Compounds | SHM a | Y1402 b | Aroma Descriptors |
|---|---|---|---|
| (Z)-3,7-Dimethyl-3,6-octadien-1-ol | - c | 280 ± 35 | Floral |
| 2-Methyl-1-propanol | - | 33 ± 5 | Sweet, wine |
| 3-Methyl-3-buten-1-ol | - | 5 ± 1 | Slightly apple-like |
| (R)-2-Octanol | - | 300 ± 23 | Creamy, cucumber |
| 3-Methyl-1-butanol | - | 444 ± 51 | Floral |
| 1-Octen-3-ol | - | 4 ± 2 | Mushroom |
| 2-Propyl-1-pentanol | - | 3 ± 1 | Mushroom, cream |
| Phenylethyl alcohol | 19 ± 3 | 304 ± 57 | Rosy, honey |
| ∑Alcohols | 19 | 1373 | |
| Dibutyl phthalate | - | 30 ± 11 | Odorless |
| (E)-9-Octadecenoic acid ethyl ester | 3 ± 1 | 5 ± 1 | Lipid |
| Dimethyl phthalate | 4 ± 1 | 2 ± 0 | Odorless |
| Hexadecanoic acid ethyl ester | 6 ± 0 | - | Cream, herb |
| Ethyl linoleate | 2 ± 0 | - | Lipid |
| ∑Esters | 15 | 37 | |
| 2,4-Dimethyl-benzaldehyde | 14 ± 2 | 85 ± 13 | Semen armeniacae amarae |
| Furfural | - | 3 ± 0 | Roasted, almond |
| ∑Aldehydes | 14 | 88 | |
| 4-Ethyl-phenol | - | 15 ± 6 | Horsy, barnyard, smoky, medical aromatic |
| 2-Methoxy-phenol | - | 13 ± 3 | Castoreum, smoke, bacon, ham |
| 4-Ethyl-2-methoxy-phenol | - | 110 ± 33 | Spicy, smoky, bacon, clove |
| Phenol | - | 6 ± 2 | Rubber |
| 2-Methoxy-4-vinylphenol | - | 920 ± 49 | Fruity, clove |
| 2,4-Di-tert-butylphenol | - | 10 ± 1 | Phenolic |
| ∑Phenols | - | 1074 | |
| 3-Methyl-butanoic acid | - | 8 ± 2 | Sour, cheesy |
| 2-Methyl-propanoic acid | - | 5 ± 1 | Cheese, dairy, buttery |
| ∑Acids | - | 13 | |
| 2,3-Dihydro-benzofuran | - | 356 ± 52 | Roast, sweet |
| Dihydro-5-pentyl-2(3H)-furanone | - | 11 ± 2 | Coconut, creamy, sweet, buttery, oily |
| 5-Hexyldihydro-2(3H)-furanone | - | 11 ± 4 | Fruity, creamy, peach, coconut, buttery, sweet |
| ∑Furanone | - | 378 | |
| 1,2-Benzisothiazole | - | 2 ± 0 | N d |
| Benzothiazole | 83 ± 6 | - | Meaty, vegetative |
| ∑Azoles | 83 | 2 | |
| 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | - | 6 ± 0 | N |
| Pentadecane | - | 2 ± 0 | Waxy |
| (3Z,5E)-1,3,5-Undecatriene | - | 7 ± 3 | Oily |
| Aniline | 5 ± 0 | - | Sweet |
| Methyl-pyrazine | 2 ± 0 | - | Roast |
| ∑Others | 7 | 15 | |
| Sum | 138 | 2980 |
Note: “a”, the volatile compounds in SHM without Y1402; “b”, the volatile compounds in SHM with Y1402; “c”, not detected; “d”, no information.
4. Conclusions
S. fibuligera, a high-yield 3-Met yeast, was selected from high-temperature Daqu. The S. fibuligera Y1402 strain showed an extensive growth pH range; a high tolerance to sugars, NaCl, nicotine, and 3-Met; and the ability to grow at temperatures as high as 40 °C. The optimal conditions for 3-Met synthesis by S. fibuligera Y1402 were determined by a single-factor experiment, Plackett–Burman design, steepest ascent test, and response surface analysis, which included a glucose concentration of 40 g/L, yeast extract concentration of 0.63 g/L, Tween 80 concentration of 2 g/L, initial pH of 5.3, fermentation time of 54 h, liquid volume of 25 mL/250 mL, inoculum size of 0.8%, L-methionine concentration of 5 g/L, shaking speed of 210 rpm, and temperature of 32 °C. Under these conditions, the yield of 3-Met reached 4.03 g/L, which is the highest among reported natural strains. In addition, the flavor substances produced by S. fibuligera Y1402 were mainly composed of compounds with floral, sweet, creamy, roasted nuts, and clove-like aromas, which have good application prospects in Baijiu brewing. In the future, researchers may focus on the construction of appropriate microbial flora with yeast Y1402 to increase the concentration of 3-Met in fermented foods like Baijiu, or production superior Daqu using the strain to boost the standard to Baijiu production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13030418/s1, Table S1: Factors and levels of a single-factor design and their optimization conditions. Table S2: Physiological and biochemical characteristics of strain Y1402. Figure S1: Comparison of 3-Met produced by yeast Y1402 and YHM-G. Figure S2: Effect of glucose concentration (10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 g/L) (a), surfactant types (control, glycerol, Tween 20, Tween 40, Tween 60, Tween 80, and Triton X-100) (b), Tween 80 concentration (0, 2, 4, 8, 16, 32, and 64 g/L) (c), L-Met addition time (0, 12, 24, 36, 48, 60, and 72 h) (d), and inoculum size (0.1, 0.2, 0.4, 0.8, 1.6, 3.2, and 6.4%, v/v) (e) on 3-Met concentration by Y1402. The same letters in the column indicate that the data do not differ significantly at 5% probability using Tukey’s test. Figure S3: The yield of ethyl acetate at different conditions at S. cerevisiae Y3401 and W. anomalus Y3604 ratio of 1:1 by simultaneous mixed fermentation in a 5 L fermenter. Note: 0, 300: the aeration rate of the fermenter is 0 vvm and the agitation speed is 300 rpm; 0.25, 300: the aeration rate of the fermenter is 0.25 vvm and the agitation speed is 300 rpm; 0.5, 300: the aeration rate of the fermenter is 0.25 vvm and the agitation speed is 300 rpm; 1.5, 600: the aeration rate of the fermenter is 1.5 vvm and the agitation speed is 600 rpm; 0, 600: the aeration rate of the fermenter is 0 vvm and the agitation speed is 600 rpm.
Author Contributions
Y.Z. carried out the experiments and wrote the manuscript. Q.S. and L.C. carried out the experiments. G.F. conceived of the study, designed the experimental protocol, and wrote the manuscript. Z.F. and C.T. revised the manuscript. J.M. and X.L. analyzed the data. R.A.B. edited the language. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the National Natural Science Foundation of China (31701592), the Beijing Natural Science Foundation (6222003), and the Open Research Fund Program of Henan Key Laboratory of Industrial Microbial Resources and Fermentation Technology (HIMFT20190205).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Seow Y.X., Ong P., Liu S.Q. Production of flavour-active methionol from methionine metabolism by yeasts in coconut cream. Int. J. Food Microbiol. 2010;143:235–240. doi: 10.1016/j.ijfoodmicro.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Che Y.X., Yin S., Wang H., Yang H.Q., Xu R.X., Wang Q., Wu Y.P., Boutet J., Huet R., Wang C.T. Production of methionol from 3-methylthiopropionaldehyde by catalysis of the yeast alcohol dehydrogenase Adh4p. J. Agric. Food Chem. 2020;68:4650–4656. doi: 10.1021/acs.jafc.0c00776. [DOI] [PubMed] [Google Scholar]
- 3.Lwa H.Q., Sun J.C., Liu S.Q. Optimization of methionol bioproduction by Saccharomyces cerevisiae using response surface methodology. Ann. Microbiol. 2015;65:197–205. doi: 10.1007/s13213-014-0850-y. [DOI] [Google Scholar]
- 4.Ma J.H., Wang Y., Zhang Y.J., Cheng L.J., Teng C., Li X.T., Fan G.S. Research progress of microbial synthesis of 3-methylthiopropanol. China Brew. 2022;41:1–7. doi: 10.11882/j.issn.0254-5071.2022.01.001. [DOI] [Google Scholar]
- 5.Etschmann M., Kotter P., Hauf J., Bluemke W., Entian K.D., Schrader J. Production of the aroma chemicals 3-(methylthio)-1-propanol and 3-(methylthio)-propylacetate with yeasts. Appl. Microbiol. Biotechnol. 2008;80:579–587. doi: 10.1007/s00253-008-1573-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang X.L., Zhang K.L., Li J.Y., Zhang Q.C., Wang C.T. Optimization of fermentation conditions for producing 3-methylthiopropanol in Saccharomyces cerevisiae by response surface methodology. J. Univ. Hebei. 2014;34:398–404. doi: 10.3969/j.issn.1000-1565.2014.04.012. [DOI] [Google Scholar]
- 7.Ma J.H., Cheng L.J., Zhang Y.J., Liu Y.C., Sun Q., Zhang J., Liu X.Y., Fan G.S. Screening of yeasts isolated from Baijiu environments for producing 3-methylthio-1-propanol and optimizing production conditions. Foods. 2022;11:3616. doi: 10.3390/foods11223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Del Castillo-Lozano M., Delile A., Spinnler H.E., Bonnarme P., Landaud S. Comparison of volatile sulphur compound production by cheese-ripening yeasts from methionine and methionine-cysteine mixtures. Appl. Microbiol. Biotechnol. 2007;75:1447–1454. doi: 10.1007/s00253-007-0971-3. [DOI] [PubMed] [Google Scholar]
- 9.Williams A.G., Noble J., Banks J.M. Catabolism of amino acids by lactic acid bacteria isolated from Cheddar cheese. Int. Dairy J. 2001;11:203–215. doi: 10.1016/S0958-6946(01)00050-4. [DOI] [Google Scholar]
- 10.Vallet A., Lucas P., Lonvaud-Funel A., de Revel G. Pathways that produce volatile sulphur compounds from methionine in Oenococcus oeni. J. Appl. Microbiol. 2008;104:1833–1840. doi: 10.1111/j.1365-2672.2007.03713.x. [DOI] [PubMed] [Google Scholar]
- 11.Hou S., Zhao L., Yang X.L., Wang C.T. Study on conditions of bio-transformation of 3-(methylthio)-1-propanol by Saccharomyces Cerevisiae SC408. J. Food Sci. Technol. 2011;29:18–22. doi: 10.3969/j.issn.2095-6002.2011.02.004. [DOI] [Google Scholar]
- 12.Wang C.T., Hou S., Zhao L., Yang X.L., Wen M.X., Sun B.G. In Application of coupling of reaction and separation technique (CRST) to biocatalytic processes of natural 3-methylthiopropanol; Proceedings of the 2010 First International Conference on Cellular, Molecular Biology, Biophysics and Bioengineering; Qiqihar, China. 21–24 December 2010. [Google Scholar]
- 13.Matthew K., Jingcan S., Shao L. Optimization of L-methionine bioconversion to aroma-active methionol by Kluyveromyces lactis using the Taguchi method. J. Food Res. 2013;2:90. doi: 10.5539/JFR.V2N4P90. [DOI] [Google Scholar]
- 14.Liu L., Yin S., Liang J.R., Wang C.T., Zhao J.X. Cloning of the aminotransferase gene ARO8 and its influence on the production of methionol in Saccharomyces cerevisiae. Sci. Technol. Food Ind. 2014;35:132–135. doi: 10.13386/j.issn1002-0306.2014.09.020. [DOI] [Google Scholar]
- 15.Deed R.C., Hou R.Y., Kinzurik M.I., Gardner R.C., Fedrizzi B. The role of yeast ARO8, ARO9 and ARO1O genes in the biosynthesis of 3-(methylthio)-1-propanol from L-methionine during fermentation in synthetic grape medium. FEMS Yeast Res. 2019;19:foy109. doi: 10.1093/femsyr/foy109. [DOI] [PubMed] [Google Scholar]
- 16.Wen M.X., Wang C.T., Yang X.L., Zhao L., Liu G.R., Tong J.M. Cloning and expression of ARO10 gene in Saccharomyces cerevisiae and it’s influence on 3-(methylthio)-1-propanol biosynthetic metabolism. Food Ferment. Ind. 2012;38:1–5. doi: 10.13995/j.cnki.11-1802/ts.2012.04.032. [DOI] [Google Scholar]
- 17.Yin S., Lang T.D., Xiao X., Liu L., Sun B.G., Wang C.T. Significant enhancement of methionol production by co-expression of the aminotransferase gene ARO8 and the decarboxylase gene ARO10 in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2015;362:fnu043. doi: 10.1093/femsle/fnu043. [DOI] [PubMed] [Google Scholar]
- 18.Yang X.L., Zhang K.L., Li J.Y., Zhang Q.C., Luo X., Wang C.T. Optimization of fermentation conditions for 3-methylthiopropanol production by recombinant Saccharomyces cerevisiae strain. J. Hebei Agric. Univ. 2014;37:94–101. doi: 10.13320/j.cnki.jauh.2014.0019. [DOI] [Google Scholar]
- 19.Du R.B., Liu J., Jiang J., Wang Y.Q., Ji X.A., Yang N., Wu Q., Xu Y. Construction of a synthetic microbial community for the biosynthesis of volatile sulfur compound by multi-module division of labor. Food Chem. 2021;347:129036. doi: 10.1016/j.foodchem.2021.129036. [DOI] [PubMed] [Google Scholar]
- 20.Frank D.C., Owen C.M., Patterson J. Solid phase microextraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT Food Sci. Technol. 2004;37:139–154. doi: 10.1016/S0023-6438(03)00144-0. [DOI] [Google Scholar]
- 21.Yang R., Liu P.X., Chang X., Xu J.Q., Yin H., Fan G.S., Teng C., Li X.T., Gong Y. Optimization of fermentation conditions for production of ethyl caproate in Baijiu using a selected isolate of Saccharomyces cerevisiae. Emir. J. Food Agric. 2021;34:59–69. doi: 10.9755/ejfa.2022.v34.i1.2808. [DOI] [Google Scholar]
- 22.Wu C.L., Li F., Ma C., Yuan Y., Fan G.S., Li X.T. Optimizing preparation conditions of cross-linked acid-stable xylanase aggregates. J. Food Sci. Technol. 2016;34:48–54. [Google Scholar]
- 23.Fan G.S., Sun B.G., Xu D., Teng C., Fu Z.L., Du Y.H., Li X.T. Isolation and identification of high-yield ethyl acetate-producing yeast from Gujinggong Daqu and its fermentation characteristics. J. Am. Soc. Brew. Chem. 2018;76:117–124. doi: 10.1080/03610470.2017.1396849. [DOI] [Google Scholar]
- 24.Buchana R.E., Gibbons N.E. Bergey’s Manual of Determinative Bacteriology. 8th ed. Science Press; Beijing, China: 1984. pp. 382–533. [Google Scholar]
- 25.Dong X.Z., Cai M.Y. Ommon Bacteria Manual System Identification. Science Press; Beijing, China: 2001. pp. 348–392. [Google Scholar]
- 26.Fan G.S., Liu P.X., Chang X., Yin H., Cheng L.J., Teng C., Gong Y., Li X.T. Isolation and identification of a high-yield ethyl caproate-producing yeast from Daqu and optimization of its fermentation. Front. Microbiol. 2021;12:663744. doi: 10.3389/fmicb.2021.663744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z.N., Yang J., Li X.S., Geng J.Z., Wu D.P. Study on biological characteristics and physiological and biochemical characteristics of Saccharomyces cerevisiae. J. Shaanxi Univ. Technol. 2022;38:39–46. doi: 10.3969/j.issn.1673-2944.2022.03.006. [DOI] [Google Scholar]
- 28.Valachova K., Horvathova V. Starch degradation by glucoamylase Glm from Saccharomycopsis fibuligera IFO 0111 in the presence and absence of a commercial pullulanase. Chem. Biodivers. 2007;4:874–880. doi: 10.1002/cbdv.200790074. [DOI] [PubMed] [Google Scholar]
- 29.Fan G.S., Xu D., Fu Z.L., Xu C.Y., Teng C., Sun B.G., Li X.T. Screen of aroma-producing yeast strains from Gujinggong Daqu and analysis of volatile flavor compounds produced by them. J. Chin. Inst. Food Sci. Technol. 2018;18:220–229. doi: 10.16429/j.1009-7848.2018.07.027. [DOI] [Google Scholar]
- 30.Chi Z.M., Chi Z., Liu G.L., Wang F., Ju L., Zhang T. Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol. Adv. 2009;27:423–431. doi: 10.1016/j.biotechadv.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z.B., Zhang K.Z., Kang Z.H., Yang J.G. Saccharomycopsis fibuligera in liquor production: A review. Eur. Food Res. Technol. 2021;247:1569–1577. doi: 10.1007/s00217-021-03743-9. [DOI] [Google Scholar]
- 32.Wang X.S., Wang B.W., Sun Z.G., Tan W., Zheng J., Zhu W.Y. Effects of modernized fermentation on the microbial community succession and ethyl lactate metabolism in Chinese baijiu fermentation. Food Res. Int. 2022;159:111566. doi: 10.1016/j.foodres.2022.111566. [DOI] [PubMed] [Google Scholar]
- 33.Yang X.C., Chen Z.J., Zhao J., Ding P.Z., Jia M.F., Gao X., Hu J.X. Breeding of acid-resistant yeast strain by UV mutation and its characteristics. China Brew. 2017;36:109–113. doi: 10.11882/j.issn.0254-5071.2017.10.023. [DOI] [Google Scholar]
- 34.Wang Q., Wang C.Y., Xiang X.Q., Xu H.L., Han G.Q. Analysis of microbial diversity and succession during Xiaoqu Baijiu fermentation using high-throughput sequencing technology. Eng. Life Sci. 2022;22:495–504. doi: 10.1002/elsc.202200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan G.S., Cheng L.J., Fu Z.L., Sun B.G., Teng C., Jiang X.Y., Li X.T. Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech. 2020;10:275. doi: 10.1007/s13205-020-02267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong L.X., Fu G.M., Liu T., Chen Y.R., Wu S.W., Cai W.Q., Xie Z.J., Wan Y. Functional microbial agents enhance ethanol contents and regulate the volatile compounds in Chinese Baijiu. Food Biosci. 2021;44:101411. doi: 10.1016/j.fbio.2021.101411. [DOI] [Google Scholar]
- 37.Xu Z.W., Wang M.Y., Tan S.Y., Wang Q., Lu G.Y., Ma J. Study on reducing nicotine content in tobacco industry waste. Tianjin Agric. Sci. 2022;28:81–86. doi: 10.3969/j.issn.1006-6500.2022.05.016. [DOI] [Google Scholar]
- 38.Landaud S., Helinck S., Bonnarme P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008;77:1191–1205. doi: 10.1007/s00253-007-1288-y. [DOI] [PubMed] [Google Scholar]
- 39.Eshkol N., Sendovski M., Bahalul M., Katz-Ezov T., Kashi Y., Fishman A. Production of 2-phenylethanol from L-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain. J. Appl. Microbiol. 2009;106:534–542. doi: 10.1111/j.1365-2672.2008.04023.x. [DOI] [PubMed] [Google Scholar]
- 40.Quek J., Seow Y.X., Ong P., Liu S.Q. Formation of volatile sulfur-containing compounds by Saccharomyces cerevisiae in soymilk supplemented with L-methionine. Food Biotechnol. 2011;25:292–304. doi: 10.1080/08905436.2011.617254. [DOI] [Google Scholar]
- 41.Peng A.A., Liu H.C., Nie Z.Y., Xia J.L. Effect of surfactant Tween-80 on sulfur oxidation and expression of sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China. 2012;22:3147–3155. doi: 10.1016/S1003-6326(12)61767-1. [DOI] [Google Scholar]
- 42.Liang Y., Zhu L., Gao M.J., Zheng Z.Y., Wu J.R., Zhan X.B. Influence of Tween-80 on the production and structure of water-insoluble curdlan from Agrobacterium sp. Int. J. Biol. Macromol. 2018;106:611–619. doi: 10.1016/j.ijbiomac.2017.08.052. [DOI] [PubMed] [Google Scholar]
- 43.Vinche M.H., Khanahmadi M., Ataei S.A., Danafar F. Optimization of process variables for production of beta-glucanase by Aspergillus niger CCUG33991 in solid-state fermentation using wheat bran. Waste Biomass Valorization. 2021;12:3233–3243. doi: 10.1007/s12649-020-01177-0. [DOI] [Google Scholar]
- 44.Hazelwood L.A., Daran J.M., van Maris A., Pronk J.T., Dickinson J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mouret J.R., Perez M., Angenieux M., Nicolle P., Farines V., Sablayrolles J.M. Online-based kinetic analysis of higher alcohol and ester synthesis during winemaking fermentations. Food Bioprocess Technol. 2014;7:1235–1245. doi: 10.1007/s11947-013-1089-5. [DOI] [Google Scholar]
- 46.Tan A., Lee P.R., Seow Y.X., Ong P., Liu S.Q. Volatile sulphur compounds and pathways of L-methionine catabolism in Williopsis yeasts. Appl. Microbiol. Biotechnol. 2012;95:1011–1020. doi: 10.1007/s00253-012-3963-x. [DOI] [PubMed] [Google Scholar]
- 47.Chursov A., Kopetzky S.J., Bocharov G., Frishman D., Shneider A. RNAtips: Analysis of temperature-induced changes of RNA secondary structure. Nucleic Acids Res. 2013;41:W486–W491. doi: 10.1093/nar/gkt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan G.S., Liu P.X., Wu Q.H., Fu Z.L., Cheng L.J., Zhu Y.T., Zhu Y.P., Yang R., Li X.T. Optimization of cultural conditions for ethyl alcohol production by Saccharomyces cerevisiae YF1914 in aerobic conditions and its aroma-producing characteristics. Sci. Technol. Food Ind. 2019;40:52–58. doi: 10.13386/j.issn1002-0306.2019.13.009. [DOI] [Google Scholar]
- 49.Liu L., Zhang C., Liang J.R., Wang C.T., Zhao J.X. Effects of knockout of gene CYS3 on metionol anabolism in Saccharomy cescerevisiae. Food Sci. 2014;35:139–143. doi: 10.7506/spkx1002-6630-201405028. [DOI] [Google Scholar]
- 50.Xu D., Zhang H., Xi J., Jin Y., Chen Y., Guo L., Jin Z., Xu X. Improving bread aroma using low-temperature sourdough fermentation. Food Biosci. 2020;37:100704. doi: 10.1016/j.fbio.2020.100704. [DOI] [Google Scholar]
- 51.Zhang J.Y., Li J.Z., Xu Y. New development direction of Baijiu enterprises-rejucenation of Baijiu consumption. China Brew. 2021;40:227–230. doi: 10.11882/j.issn.0254-5071.2021.11.041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or supplementary material.