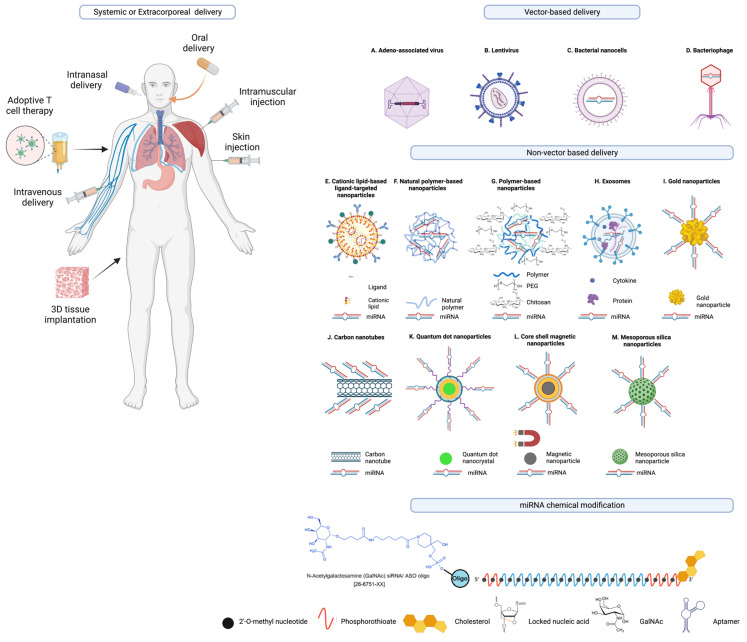
Figure 4.
Examples of miRNA delivery systems. miRNA therapeutics can be administered orally or intranasally or through venous (intravenously) or muscle (intramuscularly) or skin (subcutaneously) injections, or via cell-/tissue-directed approaches, or adoptive cell transfer, or the implantation of 3D matrices that release miRNA therapeutics, or other extracorporeal miRNA delivery strategies. Other modes of delivery of miRNA therapeutics include vector based and non-vector-based delivery systems including (A) adeno-associated virus (B) Lentivirus; (C) bacterial nanocells; (D) bacteriophages; liposomes, including monovalent and multivalent lipids such as (E) cationic lipid-based ligand-targeted nanoparticles; (F) natural polymer-based nanoparticles; (G) polymer-based nanoparticles (natural, green and synthetic, blue) conjugated with polyethylene glycol (PEG); (H) extracellular vesicles or exosomes; (I) gold nanoparticles [224]; (J) carbon nanotubes; (K) quantum dot nanoparticles; (L) core–shell magnetic nanoparticles; (M) mesoporous silica nananoparticles and others such as polymeric micelles, and mesoporous silica nanoparticles are the examples of nanocarriers as drug-delivery systems. Moreover, there have been efforts to improve the serum stability, pharmacokinetics, and tissue specificity by targeted delivery of miRNA mimics, miRNA inhibitors, and other nucleic acid therapeutics through the incorporation of various chemical modifications and/or conjugation of these RNA and nucleic acid therapeutics to biomolecules to facilitate receptor-mediated uptake such as N-acetylgalactosamine (GalNAc), 2′-O-methyl nucleotide, phosphorothioate, cholesterol, locked nucleic acid (LNA), and aptamer moieties. Created with BioRender.com (accessed on 16 January 2024).