Abstract
Cells are the fundamental functional units of biological systems and mimicking their size, function and complexity is a primary goal in the development of new therapeutic strategies. Recent advances in chemistry, synthetic biology and material science have enabled the development of cell membrane-based drug delivery systems (DDSs), often referred to as “artificial cells” or protocells. Artificial cells can be made by removing functions from natural systems in a top-down manner, or assembly from synthetic, organic or inorganic materials, through a bottom-up approach where simple units are integrated to form more complex structures. This review covers the latest advances in the development of artificial cells as DDSs, highlighting how their designs have been inspired by natural cells or cell membranes. Advancement of artificial cell technologies has led to a set of drug carriers with effective and controlled release of a variety of therapeutics for a range of diseases, and with increasing complexity they will have a greater impact on therapeutic designs.
Keywords: Artificial cells, liposomes, drug delivery, therapeutic drug delivery
1. Introduction
Cells are the fundamental functional units of living systems with assemblages of internal structures organized to support the specialized functions of growth, division, communication, and molecular transport [1–3]. Human-made cell models that simulate one or more of the complex cellular components and perform selected cellular functions can be referred to as “artificial cells”, “protocells” or “minimal cells” (Figure 1). The first artificial cell, constructed in 1957 by Chang, consisted of a vesicle made of lipids found in the cell membrane and embedded proteins [4]. With advances in materials science and a greater understanding of cellular components and their function, the science of developing artificial cells has advanced considerably. In particular the use of these cellular mimics for directed delivery and controlled release of therapies has burgeoned with advances in molecular biology, proteomics, nanotechnology, biotechnology, and polymer chemistry [5–7]. We focus our discussion on the concept of building simple functional units that recapitulate living cells, protocells, that can respond to and deliver targeted therapies.
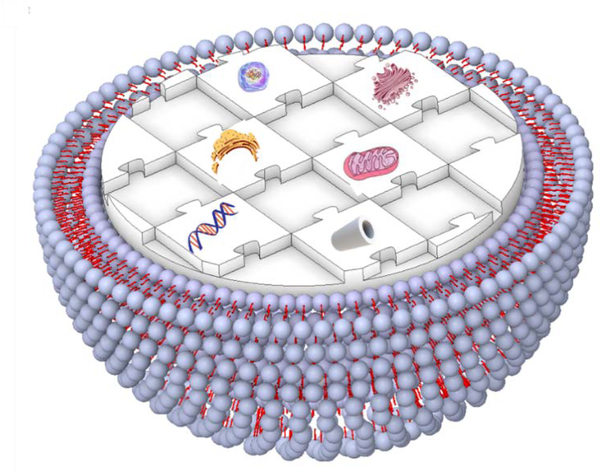
Figure 1.
Schematic illustration representing a human-made artificial cell comprising of nucleic acids, cytoskeleton, small biomolecules, and cytoplasmic organelles in a giant unilamellar vesicle.
Artificial cells have been described in many different ways in various studies, alternatively having been called “synthetic cells,” “mimicry cells,” or “protocells” [8, 9]. We will use here the term “artificial cell”, which is defined as an entity with two main features: 1) comprised of biomolecules (lipids, enzymes, membrane channels, structural proteins, etc.) that enable the entity to perform some features of living, biological cells, such as evolution, self-replication, and metabolism; and 2) produced in different structural forms using elements (polymers, combined lipid-polymer complexes, nanoparticles) that can mimic one, or more, structural or functional properties of natural cells such as transport, energy production or adaptation. In these synthetic systems, surface properties, dimensions, morphologies, and chemical responses can be modified to more closely match characteristics of natural cells, providing a simplified experimental context in which to better understand cellular functions including cell-cell interactions, and communication across distances that can inform the design of tools for biologically controlled drug delivery, which is the focus of this review [10]. These tools can be designed from a top-down or a bottom-up approach and each has an impact various features of artificial cell systems. The composition and design of artificial cells, when used as therapeutic drug delivery systems (DDS), can vary, and commonly used techniques for construction include liposomes (protocells), polymersomes, dendrimersomes, and microfluidic-based droplets. This is an emerging field with numerous opportunities as well as challenges and we discuss these in the context of existing techniques with a view toward future innovations.
2. Artificial Cell Technologies and Fabrication: From Simple Single Vesicle Structures to Complex Integrated Systems.
Cells are independent entities capable of a wide variety of interactions leading to a network of cellular centers in which controlled biochemical reactions take place within cells and are communicated throughout the network [11, 12]. An understanding of cellular dynamics and the complex structures that support integrated functions is necessary to develop artificial cells. Selecting key functions and integrating them into simple structures enables development of advanced delivery tools with broad applications in medicine. When utilizing artificial cells as bio-responsive carriers, a communication element connecting the external and internal environment mediates the sensing and responsiveness of the carrier to signals in the environment [13]. Artificial cells can encapsulate chemicals and biologics with therapeutic functions, and sets of diverse, programmed stimuli can be used to control the timing and location of their release [3, 14]. Thus, synthesis should allow for the encapsulation of biomolecules without denaturation or loss of functionality, and, for clinical applications, carriers must be biocompatible and biodegradable [15, 16]. The opportunity for fine tuning therapeutic delivery of the right amount to the right place at the right time is the benefit of artificial cells as therapeutic drug carriers. Natural cells do this every moment of every day in our bodies, and we can learn from processes how to better deliver therapeutics to where they are needed, such that we can reduce off-target, or side-effects, in the treatment of disease or restoration of function.
Both top-down and bottom-up approaches have been used to synthesize nano- and micro-sized drug carriers [8]. Carriers prepared using the top-down approach require fewer purification steps and may be easier to scale up than those using the bottom-up approach; however, carriers prepared using the top-down approach exhibit broad size distributions while those obtained by the bottom-up approach can be more easily controlled to maintain tight size distributions [17]. Understanding these synthetic approaches and their products is critical to the design and use of advanced bio-responsive drug carriers.
2.1. Top-down approach for the development of artificial cells
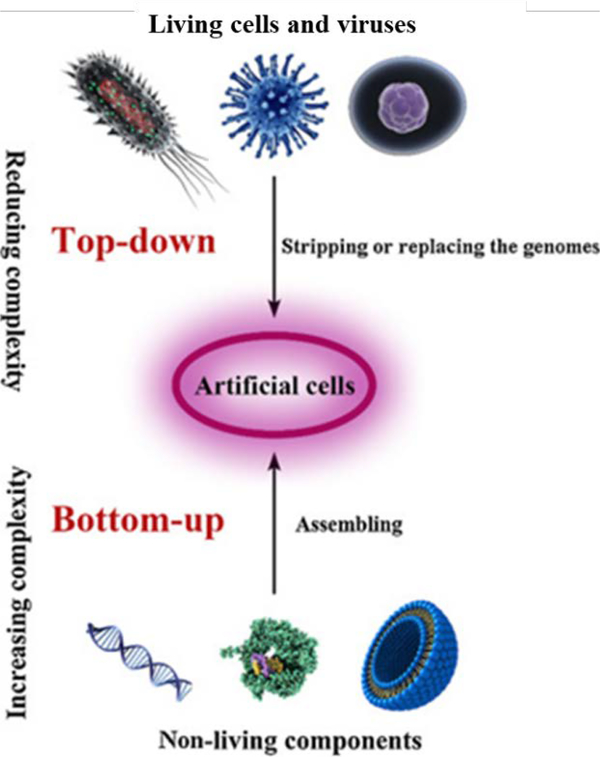
Top-down construction of artificial cells is intended to simplify the complexity of natural cells, using these cells as the starting material (Figure 2) [17]. Specifically, this approach consists of removing all cellular components that are not essential for survival of the cell [18], and can include adding back in desired functions. Methods and technologies, for stripping or replacing cellular components have been developed as part of the study of cell biology, and these approaches can be used to re-engineer cells. This top-down approach is less complicated than building a cell from scratch (i.e., from the bottom-up) [19]. Synthetic eukaryotic cells have yet to be successfully created, and therefore the top-down approach is necessary to engineer these cells, but simpler biological systems have been completely engineered. De novo synthesis of a virus that infects human cells was first reported in 2002, and there have been numerous publications using similar approaches since that initial report [20]. To create an artificial infectious poliovirus, Cello et al. synthesized full-length poliovirus cDNA which was then transcribed into infectious viral RNA with T7 RNA polymerase and used to create a poliovirus similar to natural viruses. This study showed the possibility of synthesizing more complex living organisms by chemical/biochemical methods without using a genetic template [20]. Subsequently, Venter et al. designed a computer-based genome sequence, synthesized the corresponding cDNA, stitched into the yeast genome and then transplanted the new genome into Mycoplasma capricolum recipient cells. These studies marked a technological turning point, raising the possibility of creating artificial cells and controlling organisms at the molecular level [21, 22]. The complexity of even these so-called simple systems, necessitated the use of existing cellular structures to propagate synthetic genomes in these examples of top-down designed artificial biological entities. Building from the bottom-up is a more challenging enterprise given that each component, pathway and structure would need to be engineered.
Figure 2.
The construction of artificial cells with top-down or bottom-up approaches. Adapted from Xu et al. [17], with permission from Elsevier.
2.2. A Bottom-Up Approach to Build an Artificial Cell
Recently, simplified features of living cells have been reproduced in artificial systems using nanometer- or micrometer-sized compartments [23]. In the bottom-up approach, artificial cells consist of membrane-like carriers of biomolecules that are minimally necessary to re-create specific cellular functions, generally quite simple functions like binding and membrane fusion. The bottom-up approach can yield specific bioactivities without the need to consider and manage the complex interactions among the millions of other components, processes and structures that are present in natural cells [24], but the complexity is greatly reduced. The bottom-up approach can be used to develop transfer vehicles a variety of molecular cargos including nucleic acids [25], small molecules therapeutics [26], components of vaccines [27], and enzymes [28]. In the most complex configurations, multiple components can be integrated to comprise specific biological processes.
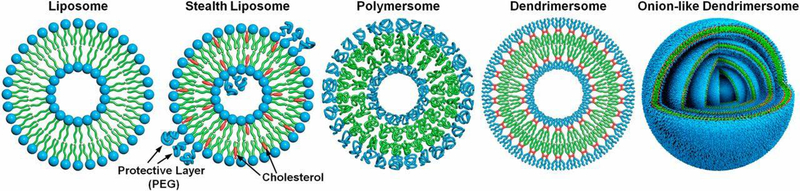
While this approach can be used to fabricate biomimetic systems, that contain nucleic acids or molecular pathways, a semipermeable outer membrane is required to enable interactions with cells or transfer of reactions into the interior of the cell where the exchange of biomolecules and ionic species occurs [19]. Currently, lipid vesicles such as liposomes, polymersomes, and dendrimersomes are used for bottom-up construction of artificial cells [6, 29, 30], and will be discussed in detail in the following sections. The preparation of water-in-water emulsion droplets using microfluidic technologies is one approach for bottom-up construction of artificial cells which provides the advantages of simple and rapid preparation, size control, and relatively small volumes. As such, microfluidic-based droplets are widely used as DDSs [31].
2.2.1. Liposomes
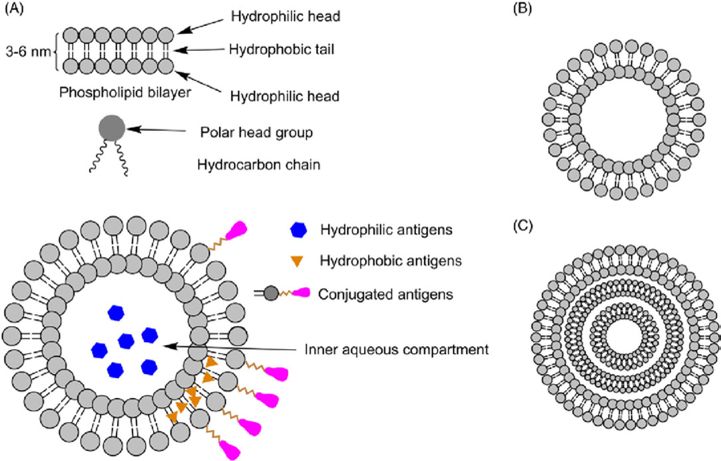
Many of the artificial cells developed as therapeutic drug carriers have been based on liposomes and comprise a number of widely used drugs including doxil—encapsulated doxorubicin [32–34]. Liposomes are spherical, supramolecular assemblies of amphiphilic molecules, including phospholipids, steroids, and polymeric materials, that constitute an aqueous core enclosed by a lipid bilayer (Figure 3A) [35]. Although, these synthetic structures do not inherently have growth, replication, communication, or other dynamic properties of natural cells, they model the lipid bilayers of living systems and have contributed significantly to the concept of artificial cells.
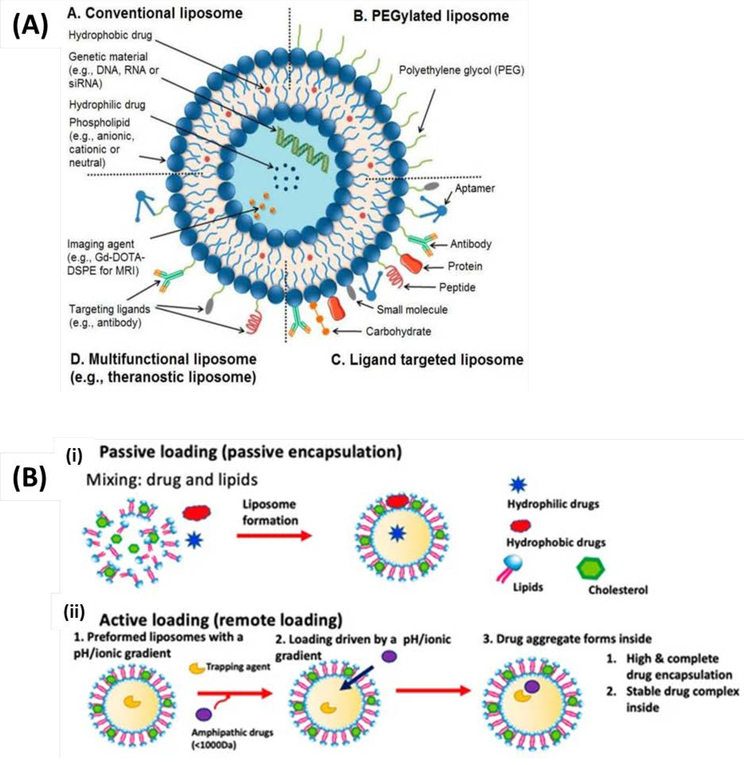
Figure 3.
(A) Liposomes: Conventional liposomes (A); PEGylated/stealth liposomes (B); ligand targeted liposomes (C); and multifunctional liposomes, (D). Reproduced from Riaz et al. [50] with permission from MDPI (B) The two major methods for liposomal drug loading. (i) Passive loading involves co-current loading and liposomal formation. (ii) In the active loading, liposomes are formed containing a gradient used to load drugs. Reproduced from Pauli et al. [47]. is an open access article distributed under the Creative Commons Attribution License.
Several different liposome preparation methods are available, including thin-film hydration or the Bangham method, solvent injection, reverse-phase evaporation, sonication, and membrane extrusion [36]. Liposomes can range from 20 nm to 10 μm in diameter. They can be classified accordingly as small (<50 nm), large (50–500 nm) or giant (10–100 μm) liposomes, depending on their size and lamellarity [37, 38]. Lamellarity, defined as the number of bilayers surrounding the liposome, and these multivesicular vesicles can encapsulate smaller vesicles. . Giant liposomes are widely used as artificial cells because their size approaches that of the eukaryotic cells, making them easily observable by light microscopy and single-level vesicle analysis methods [39]. The hydration method, which is also called the swelling method and electroformation method are the traditional methods for preparing giant liposomes and these methods are widely used as they can easily produce giant liposomes in sufficient quantities for experiments. However, in these methods, it is difficult to control monodisperse liposome size, encapsulation concentration, and asymmetry of lipid membrane [40, 41]. Researchers have also investigated methodologies for vesicle formation using microfluidics technology, aiming to create artificial cells, achieve a high encapsulation efficiency of vesicles and produce asymmetrical lipid bilayers [42]. In addition to the ability to formulate appropriate structures as in vivo drug carriers with sizes comparable to natural cells, liposomes also have the advantage of colloidal (particle) stability, [43]. Both physical stability, which is a very significant parameter for the storage and transport of a colloidal system, and chemical stability, a key parameter for predicting drug metabolism, efficacy, and toxicity, are significant for artificial cells. At the same time, it is essential that liposomes used for DDSs can be produced with precise size distributions to enable homogeneous and continuous drug release [44]. The size of particles used for drug delivery is an important consideration relative to being retained within the vasculature and targeting the endothelial cells or extravasating into the tissues, approximating the size of a cell serves to retain the entity in the blood vessel.
Liposomes can encapsulate both hydrophilic and hydrophobic drugs due to their unique core/shell structure [45]. The liposomal core can be loaded with drugs, vaccines, immunoadjuvants, or antigens through passive or active means. Passive loading involves encapsulation of target agents before or during liposome formation, while active loading takes place after liposome formation (Figure 3B) [46, 47]. Liposomes can be designed to release their cargo based on cell surface interactions, local changes in temperature or pH, or by external application of specific frequencies of light, ultrasound or magnetic fields [14].
One of the most important problems in studies on liposomes is that they collapse easily in response to environmental changes and mechanical forces due to their low bending modulus. The fragility of liposomes causes uncontrolled leakage of retained compounds and thus, complicates their use in biomedical applications and artificial cell experiments. Cell membrane and cytoskeleton are essential components that determine the shape of the living cell. Cell membrane defines the autonomy of the cell by separating the cytoplasm from the extracellular environment. Cell membranes are tolerant to environmental changes and mechanical forces. The stability of the cell membrane results from the cytoskeleton situated beneath the membrane. The cytoskeleton is a filamentous network in the cytoplasm and mechanically supports the cell membrane. The membrane is supported by actin, which forms a thin cortical shell. Based on this basic knowledge, Tsai et al. have proposed a new, facile, quick, and reproducible method to fabricate giant liposomes filled with an active actin-myosin cytoskeleton to improve mechanical strength of liposomes [48]. In another study Kurokawa et al. have constructed an artificial cytoskeleton using DNA. The DNA cytoskeleton significantly enhances mechanical stability and hence confers tolerance to osmotic shock to liposomes like the cytoskeleton in living cells. They noted that due to its biocompatibility and ease of implementing design changes, the DNA cytoskeleton could become an important stabilizer of liposomes [49].
2.2.2. Polymersomes
Polymersomes are attractive tools for bottom-up construction of artificial cells as DDSs as they have tunable membrane properties and good stability making them excellent tools to deliver a wide variety of compounds [18]. Polymersomes are synthetic analogs of liposomes being comprised of diblock or triblock copolymers that self-assemble into polymer vesicles. Compared to other polymeric carriers, polymersomes can encapsulate and deliver relatively large macromolecular payloads. Regulation of size, structure, shape, surface activity, and environmental responsiveness of polymersome-based carriers are highlighted in several recent reviews [51–53]. Polymersomes are generally more stable and less permeable than liposomes, but these properties can be modulated by the types and ratios of block co-polymers that are selected for their fabrication and the method by which they are prepared. Their properties are also determined by the size of the vesicle and the conditions under which it is stored.
Polymersomes can be produced by solvent switch methods [54], film rehydration [55, 56], solid (or bulk) rehydration [57], and electroformation [24, 58]. Formed by the self-assembly of amphiphilic molecules, polymersomes can be fabricated from several polymers, including poly(L-glutamic acid) (PGlu), poly(ε-caprolactone) (PCL), polystyrene (PS), poly(N-isopropyl acrylamide) (PNIPAM), poly(acrylic acid) (PAA) and poly(ethylene glycol) (PEG)/poly(ethylene oxide) (PEO). PEG is used frequently in the fabrication because it confers long circulation times in blood, has low immunogenicity, and has a long track record of use in human therapeutics [59]. Polymerosomes are robust versions of liposomes and are well-suited for the development of multicompartmentalized systems that mimic cells and encapsulate cellular processes.
2.2.3. Dendrimersomes
Dendrimersomes also have improved stability and versatility over liposomes and are being developed as drug delivery vehicles (Figure 4) [60]. Like their macromolecular analogs polymersomes, dendrimersomes can be used to simultaneously encapsulate both hydrophilic and hydrophobic compounds. Dendrimersomes are assembled by simple injection of a solution containing an amphiphilic Janus dendrimer, repetitively branched macromolecules with hydrophilic and hydrophobic components, into an appropriately buffered, aqueous solution [61]. Distinct from liposomes and polymersomes, dendrimersomes have superior uniformity of size, ease of formation, and high stability. Dendrimer building blocks, which are highly branched macromolecules, enable further chemical functionalization [62]. Dendrimersomes have excellent mechanical stability compared to liposomes and polymersomes while maintaining a flexibility close to that of natural cellular membrane structures. [63].
Figure. 4.
Strategies for the preparation of single-bilayer vesicles and multibilayer onion-like vesicles. Reproduced form Zhang et al. [60], with permission from the National Academy of Sciences of the United States of America.
Onion-like dendrimersomes, so-called because they have multiple superficial layers (Figure 4), have enabled development of DDSs with controlled release of encapsulated agents over time as the layers can dissolve over long periods [37]. They can consist of as many as 20 bilayers, and long-term dosing, or sequential delivery, is conceivable by enclosing the same or different therapeutic molecules within each layer [62, 64]. Despite these advantages, the mechanism by which onion-like dendrimersomes form still remains poorly understood [65], and their fabrication is a challenging and time-consuming process that limits their wide-spread use.
2.2.4. Droplet-Based Artificial Cells
The methods discussed above (Sections 2.2.1–2.2.3) for the generation of artificial cell DDSs depend primarily on traditional chemical synthesis methods (bulk synthesis methods). However, these methods are critically limited by a lack of precision, resulting in highly non-uniform size distributions of drug carriers that consequently increase the variability of drug release profiles [66]. Thus, development of methods capable of handling smaller volumes in order to provide greater control and reproducibility in the preparation of these carriers have been investigated to create systems that better recapitulate the function of living cells. Microfluidic biofabrication and biosynthesis have emerged in the fields of chemistry [67], medicine [68], biotechnology [69] and pharmacology [70], and microfluidic-based manufacturing would enable greater functionality in the development of artificial cells as droplets [71, 72]. Advanced microfabrication techniques have led to the development of devices with precisely sized microfluidic channels, which translates to a high degree of control over the formation of droplets. Changes in particle size alter the other properties like shape, surface area and porosity, which then can affect bulk properties, product performance, processability and stability. In the fabrication of any cell mimetic, monitoring particle size is paramount to the function, stability and nature of the entity and monitoring its size throughout the entire development and manufacturing process is critical.
Conventional microfluidic approaches for droplet production include T-junctions, co-flowing, and flow-focusing [73]. T-junction microfluidic devices have been used extensively since their first demonstration in the manufacture monodisperse water droplets in oil phase [74]. As shown in Figure 5A, this method employs microfluidic channels where two non-mixing liquids meet to produce droplets. The choice of the method depends on the materials used for droplets generation. For example, T-junction microfluidic devices fabricated by soft lithography methods are easily penetrated by organic solvents, leading to swelling of the channel walls. Thus, in this scenario, flow-focusing junction devices, which have two primary types as dripping and jetting (Figure 5B), that are fabricated of glass, are preferred [75]. In these devices, the dispersed phase is injected into a capillary centered in a larger diameter channel with a continuous phase flowing parallel so that co-flow occurs. Flow focusing is similar to co-flowing; however, droplet formation in flow focusing is achieved by passing the dispersed and continuous phase through a narrow opening, or orifice. The force applied by a continuous phase stream followed by a sudden decrease in the channel width facilitates droplet fragmentation and causes rapid and uniform droplet formation (Figure 5C) [75].
Figure 5.
Microfluidic geometries are used to generate droplets. (A) T-junction, (B) co-flow, (C) flow-focusing. (1: continuous phase, 2: dispersed phase).Reproduced from Zhang et al. [75], with permission from Elsevier.
To date, uni- and multi-compartmental artificial cells have been fabricated using microfluidic techniques, including using natural biomolecules [76]. Multi-compartmentalization, a distinctive feature of living cells, can provide separation and protection of the intracellular matrix and protect biological processes from unwanted interference. This biological property has inspired the development of multi-compartment vesicles. Since Schmidt et al. first reported the microfluidic-based automated production of lipid-bilayer membranes in 2006 [77], microfluidic technologies have been used to produce artificial cell membranes that can mimic morphologies and functions found in living cells, including compartmentalized structures to mimic cellular organelles [78, 79]. Such basic microfabrication techniques have provided researchers with facile methods for generating and studying lipid membranes and embedded proteins [80].
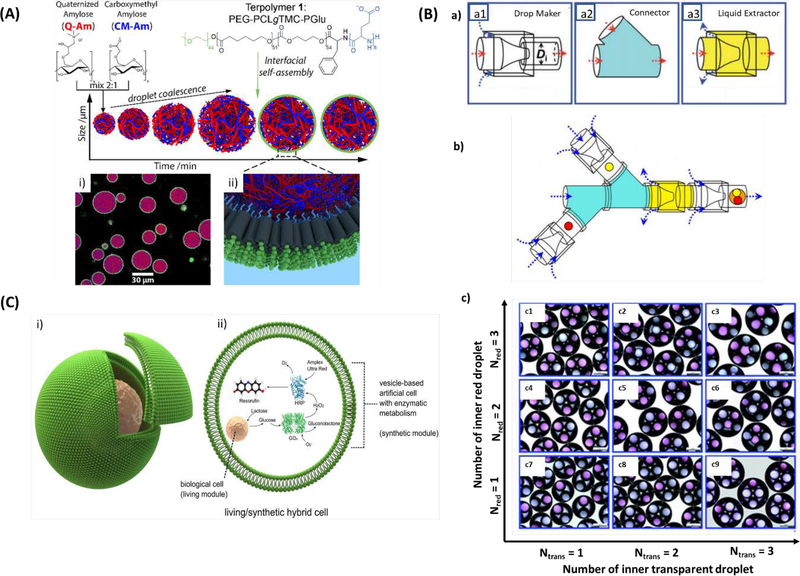
Generation of droplets with one or more mono- or hetero-compartments has been accomplished by employing the multi-emulsion method, which uses oil-water-oil (O/W/O) or water-oil-water (W/O/W) multi-emulsions [81]. However, uniformity of droplet size and fine control of the number of higher order droplets is better achieved through microfluidic methods. For example, Alexander et al. fabricated a hierarchical protocell model using interfacial self-assembly of copolymer molecules on biopolymer coacervate microdroplets by agitating the solutions with magnetic stir bars (Figure 6A). The copolymeric membranes of the developed artificial cells were naturally semipermeable, enabling incorporation of enzymatic cascades inside the membrane in response to stimuli outside of the membrane. This suggests that it may be possible to develop artificial cells that can participate in molecular communication with neighboring, non-synthetic cells and interact with varied responses depending on signals and environment [82].
Figure 6.
(A) Hierarchical self-assembly of a biodegradable triblock copolymer-stabilized cell-sized coacervate microdroplets. (i) Confocal micrograph of terpolymer/coacervate microdroplets with absorbed BSA-FITC (purple) and terpolymer membrane (green, Nile Red). (ii) 3D representation of the interfacial assembly of terpolymers. Reproduced from Mason et al.[82], with permission from the American Chemical Society. (B) (a) The microfluidic device parts to generate multicomponent multiple emulsions, (b) The schematic of the constructed microfluidic from a combination of three functional building blocks, (c) Optical micrographs of quadruple-component double emulsions exhibiting the precise control over the number and ratio of inner red and transparent droplets. Scale bars are 200 μm. Reproduced from Wang et al. [83], with permission from the Royal Society of Chemistry. (C) The construction of living/synthetic hybrid cells. (i) Diagram of a biological cell encapsulated artificial cell. (ii) The encapsulated biological cell has an organelle-like function in the vesicle reactor. Reproduced from Elani et al. [87], with permission from Springer Nature.
In another study, Wang et al. connected three different microfluidic devices to create a system to generate emulsion structures in a highly controlled manner, where droplets generated in the first device can be encapsulated in secondary droplets successively generated in the second device (Figure 6B). These emulsion structures contained distinct compartments in which unique biomolecules can be encapsulated. Multi-component emulsions offer the possibility of creating microstructures that closely resemble the organization of natural cells, where organelle compartments are suspended inside the larger cell membrane. Furthermore, this technology enables the co-encapsulation of chemically incompatible molecules (e.g. hydrophobic and hydrophilic molecules), which can be useful in the fabrication of DDSs releasing synergistic compounds or the development of microreactors to carry out biochemical reactions in a manner similar to the vectoral metabolism of living cells [83].
Lu et al. produced compartmentalized microcapsules similar to those seen in eukaryotic cells, in terms of size and shape [84]. Typical setups for droplet microfluidics use immiscible aqueous and oily phases, which could be harmful to biological systems. However, Lu et al. used an inert gas (either air or nitrogen), instead of oil, in a flow-focusing, microfluidic device to generate biopolymer-containing aqueous droplets [84]. These droplets were then converted to capsules to exploit the electrostatic complexation of oppositely charged biopolymers, along with ionic cross-linking, upon contact with a reservoir solution. This approach provides control over the number and size of the inner compartments in multicompartment capsules and, most importantly, over the contents of each compartment [84].
Bayoumi et al. synthesized an artificial cell using encapsulating the multi-compartments in the hydrogel to create vesicles. They incorporated aqueous droplets, stabilized in an oil/lipid bath, inside a hydrogel, which might serve as the basic unit for the bottom-up construction of a protocell, whose assembly could create a proto-tissue—i.e. a simple functional integrated system of interacting protocells [85]. Nuti et al. fabricated the multivesicular droplets derived cell structure for the study of compartmentalized biochemical reactions and their method demonstrated the possibility to mimic artificial organelles with optimized reaction parameters (pH, ions, etc.) in each compartment [86].
Most recently, Elani et al. used microfluidics to encapsulate living cells in vesicle-based single droplets (Figure 6C), creating a hybrid cellular bionic system whose external vesicle architecture could provide protection for encapsulated, living cells from toxic environments [87]. Encapsulated cells can act as bioreactor modules that process encapsulated substrates, which are further processed by a synthetic enzymatic metabolism co-encapsulated in the vesicle. The combination of living cells and synthetic materials using this top-down approach may enable development of more complex artificial cells as a way to overcome the inability to recreate biochemical pathways and vectoral metabolism using a purely bottom-up approach [87]. Advantages and disadvantages of the different artificial cell constructs described above are listed in Table 1.
Table 1.
Comparative overview of advantages and disadvantages of different vehicle as artificial cells.
| Vehicle | Advantages | Limitations | Reference |
|---|---|---|---|
| Liposomes | - Various sizes with either single or multiple lipid bilayers, - Diversity of lipid compositions - Simple fabrication process - Active clinical research |
- Potential complement activation and low cellular uptake - Insufficient drug loading and leakage - Premature drug release - Limited storage conditions |
[88, 89] |
| Polymersomes | - More chemical stability lifetime than liposomes - Hydrophilic core and a hydrophobic bilayer allowing encapsulation of both hydrophilic and hydrophobic drugs - Functionality for targeted and stimuli-responsive (pH, redox, enzyme, ultrasound, magnetic field, light) drug delivery |
- Residual organic solvent - Incompetent control of the early drug release - Cumbersome fabrication steps, and toxicity concerns |
[90, 91] |
| Dendrimersomes | - Modifiable branches of dendrimers - Controllable size and monodispersity - High penetration into cell membranes |
- High cost of productions - Improved quality control - Lack of clinical practice |
[92] |
| Droplet-based artificial cells | - Simple and reproducible - Amenable to automation - Narrow size distribution - Large scale manufacturing - Small volumes |
- Manufacturing and microfluidic skills required - Limitations in generating nano-sized structures |
[93, 94] |
3. Artificial Cells as Advanced Drug Carriers
3.1. Cancer Therapeutics
Cancer is one of the leading causes of death worldwide [95] and, in the United States alone, causes one in every four deaths each year [96, 97]. Chemotherapies are a major treatment strategy that target the metabolic features of cancer cells such as increased cell division and DNA replication; however, despite being characteristics of cancer, these are necessary cellular functions and delivery with a lack of specificity leads to serious off-target effects. Toxicity to healthy cells has significant negative impacts on patient health and their quality of life, necessitating the development of safer, targeted chemotherapeutics [98, 99]. DDSs based on artificial cell technologies are being developed to address this need, as they can protect drug compounds within a self-assembled core while presenting biomolecules on the surface designed to target the drug payload to tumor cells [100]. Furthermore, artificial cell-based drug carriers can be designed with unique molecular signatures, as well as drugs, for the dual purpose of therapy and diagnostics, a concept referred to as theranostics [23, 101].
The use of advanced nanotechnologies to create artificial cell-based drug carriers has enabled targeting cancer cells through: (1) primary (targeted delivery to specific organs), (2) secondary (targeted delivery to cancer cells), or (3) tertiary (targeting the uptake of DDS into specific cellular compartments) means [102]. Targeting strategies can be active, using attachment of specific ligands to the surface of a DDS that recognize and bind cancer cells, or passive, controlled by the circulation time of the DDS in the blood and its accumulation in tumor sites through leaky tumor vasculature [103–105]. With the goal of active targeting, DDSs can be modified with various surface ligands [106], most commonly antibodies or their fragments, peptides, and aptamers.
Artificial cells have the promise to function as new drug carriers with increased therapeutic efficacy and reduced risk of toxic side effects. Doxorubicin (DOX) is one of the most widely used and effective chemotherapies [107]. However, the positive charge of DOX confers a high degree of affinity for negatively charged molecules. For example, DOX binding to cardiolipin, found in the cardiac tissues, increases cardiotoxicity, which has been evidently related to its lifetime cumulative dose [108]. Therefore, artificial cell carriers are being developed to provide controlled release of DOX and cardiotoxic effects that may occur. The use of carrier formulations has been demonstrated to reduce cardiotoxicity due to lower myocardial drug concentrations [109].
The first DOX-loaded, PEG-modified liposome on the market, Doxil®, was approved by the US Food and Drug Administration (FDA) in 1995 for treatment of ovarian cancer and acquired immune deficiency syndrome-related Kaposi’s sarcoma [110]. Doxil® is marketed as Caelyx® in Europe. In 2012, a PEGylated, DOX-loaded liposome DDS, known as Lipo-dox® or Lipodox, was approved as a generic alternative to Doxil® in response to high demand for the drug. A 2012 meta-study compared controlled clinical trials evaluating free DOX or liposomal DOX and found that better patient tolerance and less toxicity effects in patients receiving liposomal DOX [111]. In 2016, Smith et al. published a study to compare toxicity, objective response rate (ORR), and time to progression (TTP) of Doxil® with Lipo-dox® when used for treatment of recurrent ovarian cancer [112]. Results showed that while Lipo-dox® and Doxil® had similar toxicities, treatment with Doxil® significantly improved ORR and TTP compared to Lipo-dox® [112]. However, the authors cautioned that additional studies of pre-clinical comparative efficacy and pharmacokinetic/tumor drug distribution studies are underway. To date, a prospective, randomized clinical study has not been published comparing Doxil® with Lipo-dox® in any other tumor type. Myocet® is a non-pegylated, DOX carrier has been approved for treatment of metastatic breast cancer in Europe and Canada since 2000, but has not been approved in the US [113]. Currently, there are a many liposome-based DDSs in U.S. clinical trials which use a variety of lipid formulations to encapsulate chemotherapies, including DOX, daunorubicin (DNR), and paclitaxel (PTX).
Like DOX, DNR is an anthracycline antibiotic drug with anti-cancer activity, but with significant side effects such as cardiotoxicity, alopecia, nausea, and vomiting [114]. As with DOX, researchers have posited that the encapsulation of DNR in artificial cell-like DDSs could be a useful approach to reduce side effects [115]. Creutzig et al. developed liposomal DNR (L-DNR), which they found in a U.S. clinical trial (NCT00111345) had increased overall antileukemic activity, and decreased treatment-related mortality when compared to treatment with idarubicin, an anthracycline antileukemic drug for the treatment of pediatric acute myeloid leukemia [116].
PTX is used for the treatment of many solid tumors such as breast, ovarian, lung and prostate cancer, but with severe acute toxicity. Compared to other chemotherapeutics, PTX has poor water solubility, making it challenging to incorporate into nanocarriers [117]. Water solubility and physical stability of paclitaxel can; however, be significantly improved by the loading into PEGylated liposomes [118]. Zhang et al. prepared PTX and gold nanoparticles (GNPs)-encapsulated liposomes (PTX-PEG@GNPs Lips), to fabricate a hybrid DDS capable of controlled co-release of multiple therapeutic molecules [119]. In mice with xenografts of HepG2 human hepatocellular carcinoma cells, this hybrid DDS led to sustained release of PTX and efficient tumor inhibition.
Over the past three decades there have been attempts to create DDSs that respond to external stimuli, such as temperature, enabling payload release in an environmentally responsive manner [120, 121]. The first study of drug release from heat-sensitive liposomes was carried out by Yatvin et al. in 1978 [122]. In 2010, Yarmolenko et al. showed that DOX-loaded, thermosensitive liposomes and mild-hyperthermia combined therapy increased median tumor growth time in mice inoculated with five different cancer cell lines (FaDu, HCT116, PC3, SKOV-3, and 4T07), with the slower-growing cancers (SKOV-3, PC-3) having the highest rates complete regressions and longest tumor growth delays [123]. In 2016, Mao et al. developed a thermosensitive, injectable hydrogel containing paclitaxel-loaded liposomes (PTX-lip), which were evaluated for the treatment of pancreatic cancer in mice (Figure 7A). The PTX-lip-gel system was retained near tumor tissue and showed no considerable toxicity to surrounding healthy tissues [124]. To visualize thermal release of small molecules from liposomes, Mackanos et al. fabricated thermal labile liposomes containing the small molecule, luciferin, as an amphipathic drug mimetic and injected these into genetically engineered mice that constitutively expressed the enzyme luciferase in all cells. When the liposomes were thermally disrupted with laser irradiation they released luciferin, and the enzymatic reaction with luciferase released photons that could be visualized in living animals as a measure of local drug delivery [125].
Figure 7.
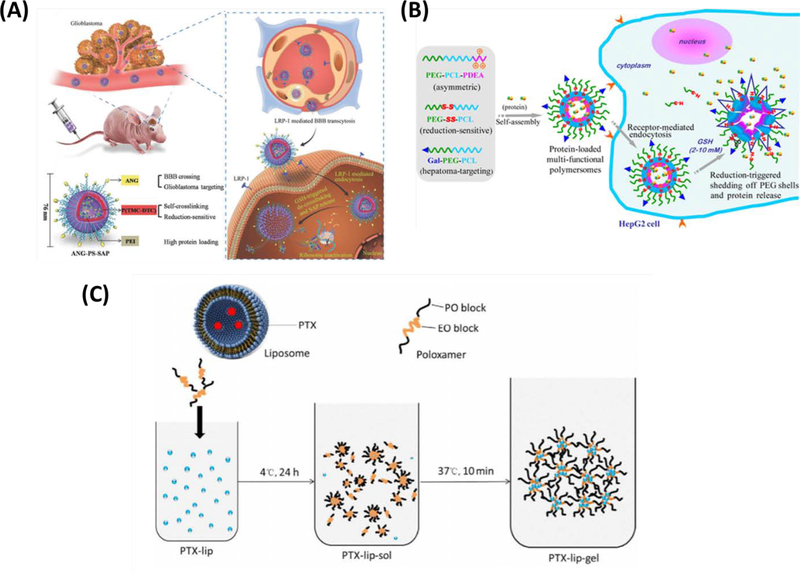
(A) Schematic presentation of ANG-directed and redox-responsive virus-mimicking polymersomes (ANG-PS) for efficient loading and selective chaperone of SAP to orthotopic human glioblastoma xenografts in nude mice. Reproduced from Jiang et al. [142], with permission from WILEY-VCH Verlag GmbH & Co. (B) Schematic illustration on hepatoma-targeting reduction-sensitive biodegradable chimeric polymersomes for active loading and intracellular release of proteins. Reproduced from Wang et al. [135], with permission from the American Chemical Society (C) The loading process of PTX liposome in the hydrogel. Reproduced from Mao et al. [124], with permission from Elsevier.
Hossann et al. demonstrated that vesicle size has a significant impact on the release of drug, specifically DOX, from thermosensitive liposomes in vitro across a clinically relevant temperature range of 30 to 45 °C. This effect was attributed to increased packaging defects in small vesicles with significantly increased membrane curvature [126]. Similarly, Hatzakis et al. showed that vesicle sizes below 150 nm increased in size with increased occurrences of packaging defects [127]. Characterization of these types of relationships have likely facilitated development liposomal chemotherapies with of temperature-controlled drug release, such as ThermoDox® which is currently in phase III clinical trials for hepatocellular carcinoma in the US [128].
The thermosensitive DDSs discussed above are generally stable at some temperature lower than body temperature, but then begin to degrade and release their contents around 37°C. Thus, the drug release mechanism is relatively passive. Alternatively, active delivery mechanisms, where an external trigger is used to induce drug release specifically at the tumor site (e.g., to which a DDS may be targeted. For example, Yan et al. developed liposome-microbubble complexes targeted to breast cancer cells [129]. They then applied focused ultrasound to the tumor area to trigger release of PTX. This approach significantly increased in vitro and in vivo antitumor efficacy in murine mammary carcinoma models. This is an attractive method of drug delivery, however, any method that depends on a priori knowledge of tumor location is limited to treating only large detectable cancers (≥1 cm3), and the undetectable small metastatic lesions will likely remain. The untreated metastatic lesions could be eliminated by an immune response that is activated by killing the primary lesion, however this cannot be predetermined, and relapse will likely occur and lead to death. Therefore, delivery methods that are directed to all cancer cells without a priori knowledge of location will be more effective.
The tumor microenvironment typically has a relatively low pH, inspiring the development of nanocarriers responsive to pH as a method to improve targeting drug release to the tumor without a priori knowledge of location [130]. In a study published in 2018, Yuba et al. developed a pH-sensitive, liposomal DDS for the chemotherapeutic agent, bleomycin (BLM) [131]. Liposomes were fabricated from egg yolk-derived phosphatidylcholine modified with N-[methoxy (polyethyleneglycol) 2000]-distearoyl phosphatidylethanolamine (PEG-PE) to achieve prolonged circulation in the bloodstream. While phosphatidylcholine is inherently pH sensitive, addition of 2-carboxycyclohexane-1-carboxylated polyglycidol-having distearoyl phosphatidylethanolamine (CHexPG-PE) provided a spacer group between the carboxyl group and ester group in the polyglycerol moiety to increased sensitivity to a pH-triggered change from a coiled-to-globule conformation when carboxylates are protonated. Intravenous administration of these liposomes lead to the suppression of growth of colorectal cancer cells (Colon-26 cells) more effectively in mice than did the free drug [131]. Bode et al. reported that cationic, PTX-encapsulating liposomes led to a reduction in the primary tumor mass and reduced angiogenesis, as compared to the use of conventional treatment, in a model of prostate cancer in rats [132].
Beyond liposomal formulations, a redox-responsive, virus-mimicking polymersome surface-decorated with Angiopep-2 peptide (ANG-PS) was developed to selectively target glioblastoma tumors and efficiently deliver the chaperone plant toxin saporin (SAP) (Figure 7B). Polymersome-based DDSs had anti-tumor activity against the U-87 MG glioblastoma cell line in vitro and efficiently delivered the drug across the blood-brain barrier with demonstrable accumulation in human xenografts of U-87 glioblastoma cells in mice [133]. Furthermore, they found that the systemic administration of SAP-loaded ANG-PS to these glioblastoma-bearing mice led only to mild side effects but had effective tumor inhibition, and significantly improved survival [133].
Protein-based drugs have unique properties relative to small molecule chemotherapeutics, including improved therapeutic specificity and reduced side effects. Liposomes and polymersomes have been used successfully for the delivery of peptides, synthetic lipopeptides, or proteins as cancer therapeutics. For example, asparaginase-encapsulated liposomes were reported to improve survival in a mouse model of lymphatic leukemia [134]. Similar to how studies of targeted chemotherapeutics have found that liposome encapsulation mitigates systemic toxicity, Gasper et al. showed that liposome encapsulation prevented the production of anti-asparaginase antibodies in animals treated with asparaginase-encapsulated liposomes. In another study, Wang et al. designed chimeric biodegradable polymersomes targeting hepatocellular carcinoma, where drug release is triggered by the reducing environment of the cytoplasm (Figure 7C) [135]. Galactose-PEG-PCL, PEG–PCL–poly(2-(diethylamino)ethyl methacrylate) (PEG-PCL-PDEA, asymmetric), and PEG-SS-PCL polymersomes were loaded with bovine serum albumin (BSA) and ovalbumin (OVA) as model biological therapies, or cytochrome C or granzyme B as potential therapeutics. In vitro studies showed that therapeutic proteins released by chimeric polymersomes could induce apoptosis of the HepG2 human hepatocellular carcinoma cell line better than free protein [135]. Furthermore, addition of galactose to polymersome surfaces enabled targeting of protein-loaded polymersomes to hepatocellular carcinoma cells through binding to the asialoglycoprotein receptor [135].
In addition to galactose, various molecules have been explored as tumor-targeting moieties for the decoration of nanosized delivery systems, including anisamide, a benzamide derivative, which shows high affinity to sigma receptor. The sigma receptor is a membrane bound protein known to be overexpressed on several human malignancies, including those in lung and prostate cancers [136]. Anisamide-targeted, PEG-based liposomes delivered DOX to DU-145 prostate cancer xenografts in mice and effectively inhibited tumor growth with minimal toxicity [137]. Free DOX was also effective at inhibiting tumor growth, but had significant toxicity. Lu et al. developed pH-sensitive degradable chimeric polymersomes decorated with anisamide to target lung cancer. When loaded into PEG-b-poly(2,4,6- trimethoxybenzylidene-1,1,1-tris(hydroxymethyl)ethane methacrylate)-b-PAA (Anis-PEG-PTTMA-PAA) and PEG-PTTMA-PAA polymersomes, granzyme B release was accelerated under mildly acidic conditions (pH:5) [138]. Furthermore, anisamide appeared to effectively target sigma receptor-overexpressing cells, including H460 lung cancer cells and PC-3 prostate cancer cells, through which polymersomes were quickly internalized. In another example of targeting specific tumor cell receptors, Linuma et al. investigated the therapeutic effects of DDS consisting of transferrin-modified PEG liposomes, to target human gastric adenocarcinoma cells, and encapsulated cisplatin, a common chemotherapeutic, in a mouse mode of xenografted MKN45 cells [139]. Their results showed that these liposomes might be useful in the treatment of gastric cancer with peritoneal spread [139].
Many biotherapeutics are inherently sensitive to their surroundings and susceptible to biodegradation. Such agents require tailored DDSs. For example, Tran et al., developed a microfluidic-based methods for highly efficient production of amphiphilic, heparin-folate-retinoic acid (HFR) bio-conjugates for selective and efficient delivery of the drug to cancer cells in vitro [140]. Another anti-tumor agent requiring an alternative liposome formulation because of rapid changes in bioavailability and frequent side effects is all-trans retinoic acid (ATRA). Cristiano et al. developed a liposomal carrier for ATRA and reported greater anti-tumor activity, compared to the free drug, on two out of three thyroid carcinoma cell lines (FRO and B-CP, but not PTC-1) after 24 h of incubation in vitro [141].
Artificial cells are in development with the dual purposes of tumor imaging/identification and treatment. Belwal and Singh developed a nano-sized, silica-supported PEG liposome for the controlled release of vincristine sulfate, a chemotherapeutic drug used to treat leukemia [143]. In addition to vincristine sulfate, these protocells could be loaded with various dyes to provide different color contrast in during tumor imaging. Notably, addition of silica supports to PEG liposomes increased residence times in circulating body fluids, and thus the number of protocells reaching tumors. Dagar et al. modified stabilized liposomes with vasoactive intestinal peptide (VIP), receptors for which are overexpressed in breast cancer cells, and loaded them with both the chemotherapeutic n-methyl nitrozourea (MNU) and the radionucleotide Tc99m. These DDSs were able to actively target breast cancer, delivering MNU and providing a contrast agent for nuclear imaging [144]. Finally, DDSs have been developed to facilitate photodynamic therapy (PDT) for cancer, where cancer cells are selectively heated using a combination of a photosensitive reagent and applied light [145, 146]. Theranostic approaches enable monitoring delivery of the therapeutic agent and often times can be used to monitor therapeutic outcomes such that image guidance can be used to optimize therapy and determine efficacy.
3.2. Nucleic acid-based therapy
Nucleic acids can be used as therapies, and quite recently as vaccines, and have shown promise for treatment of several human diseases, cancer [147], viral infections [148], and diseases that result from genetic disorders [149]. However, since nucleic acids are positively charged, unstable and targeted for degradation by multiple cellular mechanism complex delivery strategies are necessary for their effective use as therapeutics. Artificial cell-based systems seem ideal for nucleic acid delivery, and have been investigated for the delivery oligodeoxynucleotides, plasmid DNA (pDNA), ribozymes, siRNA, and miRNA [150–152]. Efficiency of targeted delivery of nucleic acid-based therapeutics depends on size, lipid-to-nucleic acid ratio, chain length, and surface charge, the presence of ester bonds, and nature and chain length of ligand composition and their clinical utility will be determined by all of these features [46].
Poor lipid solubility and electrostatic repulsion generally prohibit nucleic acids from spontaneously crossing lipid-rich, negatively charged cell membranes. Thus, design of efficacious carriers for delivery of nucleic acids is essential and a focus of intense investigation [153]. Owing to their opposite surface charge, cationic liposomes interact strongly with negatively charged DNA and RNA molecules and act as good carriers, but with nucleic acids, delivery alone can be inadequate if the nucleic acid must be transcribed and/or translated into their functional macromolecules. DNA-loaded liposomes are positively charged so that they avoid the electrostatic barrier faced by naked DNA to enter natural cells [154]. In addition, the micellar structure of liposomes sequesters water-soluble nucleic acids to the center, leaving lipid-soluble surface groups which can pass through a cell membrane.
The first cationic pDNA-liposome system was developed by Felgner et al. in 1987 [155]. They demonstrated improved DNA transfer, also known as transfection, efficiency by utilizing a synthetic cationic lipid, N- [1- (2,3-dioleyloxy) propyl] -N, N, N-trimethylammonium chloride (DOTMA), as a unilamellar liposomal carrier. DOTMA facilitated the fusion of the lipid-DNA complexes with the plasma membrane of cultured cells, resulting in both uptake and expression of DNA. Since then, many liposome-gene complex systems have been developed and optimized for use in a large number of delivery applications. Phospholipids commonly used for the stabilization of the liposome/DNA complexes include dioleoil phosphatidylethanolamine (DOPE) [156], dioleoyl phosphatidyl choline [157], DC-cholesterol HCl (3β- [N- (N′, N′-dimethylaminoethane)-carbamyl] cholesterol hydrochloride) [158], DOTAP (1,2-dioleoyl-3-trimethylammonium-propane [chloride salt]) [159], DOBAQ (N- [4-carboxybenzyl] -N, N-dimethyl-2,3-bis (oleoyloxy) propan-1-aminium) [160], DDAB (dimethyldioctadecylammonium [bromide salt]) [161] and MLV5(N1-[2-((1S)-1-[(3-aminopropyl)amino]-4-[di(3-amino-propyl)amino]butylcarboxamido)ethyl]-3,4-di[oleyloxy]-benzamide) [162]. The ability of cationic liposomes to complex with nucleic acids to increase their ability to transfect animal target cells has led to their pervasive use for non-viral gene delivery. Double stranded nucleic acid is more stable than single stranded nucleic acid, and the prevalence of ribonucleases that cut RNA can limit the use of RNA as a therapy—protecting the nucleic acid while also delivering a functional molecule is a challenge and a number of successful strategies have been reported.
The design and synthesis of biodegradable polymeric materials that provide suitable polymeric carrier properties for temporal and spatial distribution of nucleic acids in the body have been described [163, 164]. Polymeric carriers targeting based on particle size and surface charge represent a critical issue for material selection and design; however, the release of the active agent will also depend on the distribution in the carriers and the rate of degradation of the polymer. An ideal gene delivery system should show high transfection levels for long-term administration, be non-toxic and biodegradable. Polyethyleneimine (PEI) is one of the most effective cationic compounds used for nucleic acid delivery into mammalian cells [163]. In 2009, Koh et al. developed PEI/pDNA complexes using a microfluidic, hydrodynamic focusing technology to efficiently condense pDNA. Compared to bulk mixing, this method improved size control, transfection efficiency, and cytocompatibility of the PEI / pDNA complexes [164]. This method was particularly useful for assembling complexes with small plasmids and oligonucleotides, which are less susceptible to chain breakage at higher flow rate ratios.
Using pH-sensitive poly(2-(methacryloyloxy)ethylphosphorylcholine)-co-poly(2-(diisopropylamino)ethylmethacrylate) (PMPC–PDPA) diblock copolymers, Lomas et al. developed polymersomes for encapsulation and delivery of pDNA as a non-cytotoxic synthetic vector for the rapid and efficient intracellular delivery of active agents [165, 166]. DNA-loaded polymers have biomimetic surfaces due to the presence of PMPC chains and minimize any interaction with blood plasma proteins and hence prolonged the average polymer circulation time in the blood. The PMPC-PDPA polymers are able to retain encapsulated DNA for at least two weeks, indicating that their membranes have a very low degree of “leakage”. Also, unlike cationic liposome transfection agents, PMPC-PDPA polymers led to very low cytotoxicity and significantly reduced levels of cellular damage [165, 166].
Small interfering RNA (siRNA) and siRNA mimetics have the advantages of being inherently functional without needing to be translated into proteins, and being catalytic offering efficiency for turning genes and pathways on or off. As such, these molecules are potentially useful therapeutic tools for silencing disease associated genes or activating beneficial pathways, however, use of any nucleic acid is limited by instability. The challenges of using siRNA as a therapy are maintaining stability in the circulation, directed delivery to the target cells, and effective release within the target cell for function. Discher and colleagues showed delivery of siRNA and antisense oligonucleotides using biodegradable block copolymers, including PEG-b-PCL, PEG-b-PLA, and PEG-b-PBD-based non-ionic polymersomes [167]. By varying the copolymer molecular mass, the mechanical properties and permeability of the polymersomes can be adjusted, and generally they circulate in vivo for much longer time than lipid vesicles and cationic carriers. The results indicated that non-ionic polymer vesicles are both biocompatible and immunocompatible, offering advantages over viral-mediated delivery of siRNA encoded as short hairpin RNA (shRNA) or use of cationic compounds.
While targeted delivery can selectively and preferentially deliver therapeutic agents to disease sites to improve therapeutic efficacy, the reticuloendothelial system comprised of phagocytes and the renal clearance pathway are frequent dead end destinations of systemically delivered nanoparticles, and represent the greatest obstacles to successful delivery [168]. Wilhelm et al. analyzed 117 published studies that reported the delivery efficiency of various nanoparticles to tumors [169]. Overall, a median of about 0.7% of the injected nanoparticles reached their targets. Moreover, the percentage of nanoparticles reaching cancer cells in vivo maybe be even less than 0.7% because of the dense tumor stroma. Addressing this low efficiency is a central challenge for translating nanomedicines into the clinic.
Extracellular vesicles (EVs), which are natural cell-derived vesicles important for intercellular communication between adjacent and distant cells in the body [170–174], and they present the opportunity to create artificial cells as DDSs that overcome these issues with synthetic nanoparticles. EVs have been shown to be traverse dense extracellular matrix (ECM) environment, while liposomes or synthetic nanoparticles with similar sizes cannot diffuse through the ECM [175]. Deformability of EVs appears to be essential for their superior ability to transverse tissue [176]. The naturally produced lipid bilayer membrane of EVs varies in composition from the cell membrane, rendering them highly stable [177]. In addition to the advantages of good tissue penetration and stability in circulation and tissues, biocompatibility and low immunogenicity have led researchers to explore the use of EVs as nanocarriers for various cargo therapeutics, from chemotherapeutics to genes [178–180].
EVs can be engineered to carry therapeutic cargo, including proteins, drugs, or nucleic acids (miRNA, DNA), using active and passive bottom-down approaches. In active approaches, therapeutic cargo can be incorporated into donor cells, and subsequently packaged into EVs, through co-incubation with drugs or transfection [181], and loaded EVs can then be isolated. In passive approaches, EVs are isolated prior to loading with therapeutic cargo using various physical methods including incubation, sonication, electroporation, extrusion, permeabilization, and freeze-thaw cycling [181–183]. EVs are also uniquely suited as a platform for development of targeted therapies since they are produced with cell receptors that interact with cells and sometimes preferentially with specific cell types [184–186]. Additional engineering of EVs, such as hybrid carriers fusing liposomes with EVs [187–189] or surface decoration with PEG [190], can be performed to potentially improve delivery efficiency and circulation times. Despite the exciting potential of EVs as clinical therapeutics, additional work is needed to fully characterize their biodistribution and pharmacokinetics as well as to achieve controlled, reproducible EV production [191].
The limited number of EVs produced by cells hampers their applications. Therefore, artificial EV-mimetics have been generated and investigated as a therapeutic tool for drug delivery [192]. However, most of the artificial EV-mimetics proposed or studied to date are actually liposomes. Small unilamellar vesicles (SUVs) are ideal precursors for the preparation of vesicles that can mimic exosomes due to their similarities to natural exosomes (size range and membrane disposition). Lu et al. developed exosome-mimicking liposomes for delivery of VEGF siRNA to A549 cancer cells and HUVECs. These exosome-mimetics had lower cytotoxicity and higher storage and physical stability (reduced aggregation) in serum. It was also reported that they can endocytosis into A549 cells and HUVECs, and their oligonucleotide delivery efficiencies are much lower compared to those of cationic lipids such as Lipo-2000 and DOTAP [193].
3.3. Vaccine Delivery
Vaccines evoke immune responses in patients leading to the production of antibodies and protection against future disease [194]. Vaccine delivery systems based on artificial cells may offer significant improvements to more traditional systems. Traditional vaccines generally contain inactivated or attenuated disease-causing agents, such as antigenic proteins of pathogens, which are generally associated with toxicity, allergic responses, hypersensitivity, or inflammation [194]. Subunit vaccines, are prepared from a fragment of the pathogens (e.g. carbohydrates, recombinant DNA, RNA or synthetic proteins) and reduce the danger of inducing the disease by living viruses or bacteria as vaccines [195]. Liposomes and their derivatives have intensively been studied as potential drug carriers for the effective delivery of peptides as antigenic subunits [194–210]. Liposomes can also improve the stability of antigens by providing protection against enzymatic degradation, thus reducing the required dose [211]. Antigens can be encapsulated within the liposomes at different stages during liposome preparation [198, 212] or, alternatively, covalently conjugated [195] or physically adsorbed [198, 202] to the liposomal surface. The method by which antigens are incorporated can have distinct effects on their immunogenic potential. For example, Quer et al. reported that the adsorption of hemagglutinin antigens to a liposomal surface was more effective in evoking an immune response than antigenic encapsulation inside liposomes [198]. More recently Lanza et al. demonstrated that Leishmania hypothetical (LiHyp)-liposomal vaccines incorporated into a dissolvable microneedle patch provide unprecedented control over antigen release and long-term protection against parasitic leishmaniasis infection [210].
The composition of liposomes is also important in inducing immune responses to antigens [213, 214]. Phillips et al. showed that liposomes prepared from phosphatidylethanolamine (PE) or phosphatidyl glycerol (PG) were more effective than phosphatidylserine (PS)-based liposomes in inducing immune responses [213]. More recently, Kaur et al. showed that the presence of cholesterol in liposomes increases bilayer rigidity, resulting in prolonged residence in vivo [215]. However, Nakano et al. showed that cholesterol in liposomes can adversely affect the immunoglobulin (IgG) response [216], indicating that cholesterol may also reduce the immunogenicity of liposomes [195].
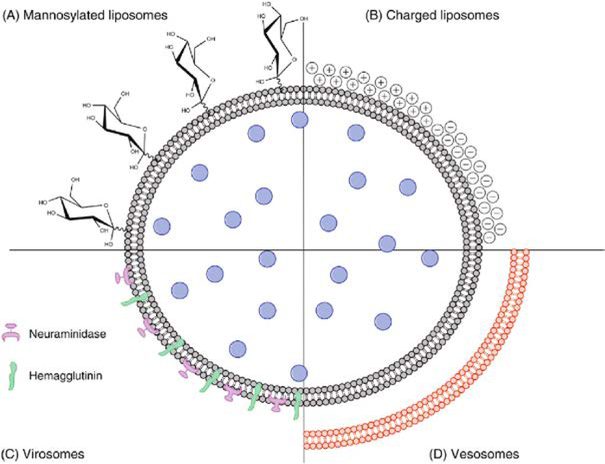
As liposomes include an inner aqueous compartment and hydrophobic outer surface (Figure 8A–C), they are capable of incorporating both hydrophilic or hydrophobic components; for example, to create dual antigen delivery [194]. In another example, the outer membrane of liposomes can be modified with APC-targeting molecules, such as mannose to facilitate antigen delivery (Figure 9A) [195, 207, 208]. The surface charge also plays a critical role in liposomal interactions with APCs (Figure 9B). Conventional wisdom indicates that cationic liposomes are more likely to interact with anionic cell membranes and efficaciously deliver the antigen payload into cells into antigen-presenting cells (APCs; e.g., macrophages and dendritic cells), evoking a stronger immune response. However, some studies have shown a higher efficacy of negatively charged liposomes to induce Th1- and Th2-biased cellular immune responses, implicating other factors, such as the type of phospholipids on liposomes, may substantially affect APC-liposome interactions [195]. Other liposome-based vaccine delivery systems include, but are not limited to, virosomes and vesosomes. Virosomes are unilamellar liposomes based on viral membrane proteins and have been used widely for vaccination against hepatitis A and influenza (Figure 9C). Vesosomes, or “double-bagged liposomes”, have dual phospholipid bilayers to provide enhanced protection of encapsulated antigens (Figure 9D) [195]. Boyer et al. showed that the external membrane of vesosome can provide a physical barrier to protect the internal membrane from degradation, thus increasing the drug retention time from minutes to hours [217].
Figure 8.
(A) Schematic illustration of liposomes as a vaccine delivery system. (B) Unilamellar liposomes. (C) Multilamellar liposomes. Reproduced from Marasini et al. [195], with permission from Elsevier.
Figure 9. Schematic presenting various types of liposomes for vaccine delivery.
(A) Modified liposome with a targeting moiety (e.g. mannose). (B) Cationic or anionic liposomes, (C) virosomes, and (D) vesosomes Reproduced from Marasini et al. [195], with permission form Elsevier.
Polymerization of liposomal nanocarriers can improve their stability under physiological conditions. For example, vaccines for SARS-CoV2 (the virus that causes COVID-19) that have recently developed by Pfizer and Moderna and widely distributed among the human population, contain an mRNA that encode a viral subunit that is wrapped in liposomal nanoparticles, which are then coated by a layer of PEG to increase their residence times in vivo [218, 219]. The challenge of using mRNA as a therapy or vaccine is that it must be delivered to the cytoplasm of cells in a way that it can escape the endosome and be translated into protein. This presents a significant challenge that was overcome by this double coating approach by Pfizer and Moderna. An alternative approach is to use charge-altering releasable transporters (CARTS) where the encapsulated mRNA has a net charge that allows binding and internalization by the cells, and then the charge changes to escape the endosome and release functional mRNA that can be translated into functional proteins [220].
A wide variety of polymerized liposomes have been developed as oral or nasal vaccines as they exhibit high stability, controlled antigen release and mucosal antiviral responses in mice immunized with these liposomes [197, 201, 204, 209]. Beyond liposomes, other types of vesicle-based artificial cells such as polymersomes have also shown some potential for vaccine delivery. For example, polymersome nanocarriers have been shown to enhance the immunogenicity of influenza subunit vaccine at in vivo [221] and humoral immunity against Lassa virus (LASV), which is responsible for severe hemorrhagic fever with high mortality [222]; however, these polymeric nanostructures have not reached clinical trials [223].
Subunit vaccines made from highly purified antigens often show poor immunogenicity, however, subunit vaccines exhibit some distinctive structural features, particularly defined as the pathogen/damage/danger-related molecular patterns (PAMPs) exhibited on microbe surfaces. To overcome the poor immunogenicity, subunit vaccines are often combined with an adjuvant or included in a carrier to direct antigens to APCs and enhance the immunostimulatory effect [194]. These adjuvants may include liposomes, emulsions, polymeric and inorganic nanoparticles, or pattern recognition receptors such as squalene, saponin and insoluble aluminum salts [224, 225]. Liposomes have long been validated as intrinsic adjuvants which, unlike most adjuvants, have minimal reactogenicity [226]. Other advantages of liposomal adjuvants are biodegradability, good biocompatibility and controlled release of encapsulated payloads [227]. Liposomes can exert strong immunomodulatory effects due to their particulate structure and their ability to interact with APC lipid receptors through targeting molecules on their surfaces, thus fusing with the wall of target cells and getting rapidly incorporated into the reticuloendothelial system [194]. Liposomes can also be used to encapsulate structurally different epitopes to induce various immune responses [228–230]. For example, Cole et al. showed that TLR7/8 and TLR4 agonists co-encapsulated liposome delivery systems used with a split-flu vaccine resulted in stronger immune responses against heterologous influenza infections [203].
4. Perspective and Conclusion
As engineered biological systems, artificial cells can be developed to offer unique opportunities for drug delivery with improved safety and efficiency. An artificial cell can be fabricated using either top-down or bottom-up approaches, where each strategy has its advantages and limitations. Among the artificial cell fabrication techniques, microfluidic-based methods represent one of the most advanced tools for bottom-up assembly of artificial cells. In general, bottom-up production of artificial cells as DDSs, especially with the use of microfluidic systems, is expected to lead to the development of autonomous microcarriers with narrow polydispersity that can monitor their environment and intervene when necessary. However, DDSs currently approved for clinical use are typically made using traditional emulsion methods, because microfluidic platforms suffer from the limited quantity of synthesized particles that they can produce [231, 232]. Alternatively, EVs provide a promising platform for top-down fabrication of artificial cells. In general, bottom-up approaches have been more successful. Artificial cells that use liposomes, polymersomes, or dendrimersomes to create a membrane component have been developed as advanced DDSs for cancer treatments, gene therapies, and vaccines. Surface modification of these carriers can improve payload delivery, through increasing circulation times and preventing degradation, and enable systems with active-targeting and stimulus-responsive capabilities. Overall, artificial cells can be fabricated with various sizes, deformability, sustained/controlled payload release profiles, and versatile targeting functions.
However, most attempts to create artificial cells have focused on recreating biological-like activity under laboratory conditions in the absence of other living cells. But a few laboratories have begun to put together more complex ecosystems of different artificial cells or artificial cells mixed with natural living cells [233, 234]. Artificial cells that integrate with the wider cellular community can help reveal the physicochemical underpinnings of cellular behavior and provide opportunities for designing advanced drug delivery systems. Therefore, despite the potential and future implications of this technology, further studies on the interaction of artificial cells with eukaryotic cells under physiological conditions are needed.
Other promising approaches to consider as DDS are Cellular hitchhiking [235, 236] and cell-particle hybrid systems [237, 238]. Cellular hitchhiking method involves nanoparticle attachment to circulating blood cells to improve the delivery capabilities of the nanoparticles (e.g. targeting and circulation). Unfortunately, these delivery systems are still in their inception and therefore not generally used and much less researched, but systems developed and tested in preclinical settings have demonstrated improved targeting and stealth capabilities over their chemically and physically modified counterparts. The combination of these strategies with artificial cells could pave the way for the development of new DDSs.
Despite extensive progress over the last few decades, before artificial cells capable truly dynamic and interactive relationships with natural biological systems are created, important questions must be answered regarding: (i) communication between artificial cells and their local environment; and (ii) interactions between different types of artificial cells with different functions. There are still remarkable differences between the living cellular membranes and their artificial compartments, and studies to develop new materials as well as basic experiments to better understand how a membrane’s composition contributes to its physical properties and biological functions are important to bridge this gap. In fact, artificial cells will likely facilitate these basic biological studies. To date, a limited number of materials have been used for the production of artificial cells. Therefore, the development of alternative and new smart and improved properties such as nanoscale efficiency, self-organization, and adaptability for therapeutic and diagnostic applications is needed. Ultimately, creating artificial cells with properties and functions of living cells and have broad clinical utility will require extensive cross-disciplinary cooperation among the fields of biophysics, biochemistry, medicine, biomedical engineering, materials science and molecular/cellular biology.
Acknowledgements
The authors have no competing interests. We acknowledge funding from National Institutes of Health (1UG3TR003148-01, N.A.), the American Heart Association (COVID-19 Rapid Response Award 20203858, N.A.), and generous support from the James and Kathleen Cornelius Endowment (C.H.C). S.E.D. would like to thank the Scientific and Technological Research Council of Turkey (TÜBİTAK) for financial support. M.T. would like to acknowledge financial support from Fonds de recherche du Québec - santé (FRQS).
REFERENCES
- 1.Marth JD, A unified vision of the building blocks of life. Nature cell biology, 2008. 10(9): p. 1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noireaux V, Maeda YT, and Libchaber A, Development of an artificial cell, from self-organization to computation and self-reproduction. Proceedings of the National Academy of Sciences, 2011. 108(9): p. 3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szostak JW, Bartel DP, and Luisi PL, Synthesizing life. Nature, 2001. 409(6818): p. 387–390. [DOI] [PubMed] [Google Scholar]
- 4.Chang T, Report on “method for preparing artificial hemoglobin corpuscles”. 1957.
- 5.Blain JC and Szostak JW, Progress Toward Synthetic Cells. Annual Review of Biochemistry, 2014. 83(1): p. 615–640. [DOI] [PubMed] [Google Scholar]
- 6.Dzieciol AJ and Mann S, Designs for life: protocell models in the laboratory. Chemical Society Reviews, 2012. 41(1): p. 79–85. [DOI] [PubMed] [Google Scholar]
- 7.Mansy SS, et al. , Template-directed synthesis of a genetic polymer in a model protocell. Nature, 2008. 454(7200): p. 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Ruder WC, and LeDuc PR, Artificial cells: building bioinspired systems using small-scale biology. Trends in Biotechnology, 2008. 26(1): p. 14–20. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Wu F, and Tan C, Synthetic Biology: A Bridge between Artificial and Natural Cells. Life (Basel, Switzerland), 2014. 4(4): p. 1092–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salehi-Reyhani A, Ces O, and Elani Y, Artificial cell mimics as simplified models for the study of cell biology. Experimental Biology and Medicine, 2017. 242(13): p. 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janmey PA and McCulloch CA, Cell mechanics: Integrating cell responses to mechanical stimuli, in Annual Review of Biomedical Engineering. 2007. p. 1–34. [DOI] [PubMed] [Google Scholar]
- 12.Bimbo LM, et al. , Toxicological profile of therapeutic nanodelivery systems. Current Drug Metabolism, 2012. 13(8): p. 1068–1086. [DOI] [PubMed] [Google Scholar]
- 13.Neven KY, Nawrot TS, and Bollati V, Extracellular Vesicles: How the External and Internal Environment Can Shape Cell-To-Cell Communication. Current Environmental Health Reports, 2017. 4(1): p. 30–37. [DOI] [PubMed] [Google Scholar]
- 14.Chandrawati R and Caruso F, Biomimetic Liposome- and Polymersome-Based Multicompartmentalized Assemblies. Langmuir, 2012. 28(39): p. 13798–13807. [DOI] [PubMed] [Google Scholar]
- 15.Aufinger L and Simmel FC, Establishing Communication Between Artificial Cells. Chemistry – A European Journal, 2019. 25(55): p. 12659–12670. [DOI] [PubMed] [Google Scholar]
- 16.Buddingh’ BC and van Hest JCM, Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity. Accounts of Chemical Research, 2017. 50(4): p. 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Hu S, and Chen X, Artificial cells: from basic science to applications. Materials Today, 2016. 19(9): p. 516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roodbeen R and van Hest JCM, Synthetic cells and organelles: compartmentalization strategies. BioEssays, 2009. 31(12): p. 1299–1308. [DOI] [PubMed] [Google Scholar]
- 19.Supramaniam P, Ces O, and Salehi-Reyhani A, Microfluidics for Artificial Life: Techniques for Bottom-Up Synthetic Biology. Micromachines, 2019. 10(5): p. 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cello J, Paul AV, and Wimmer E, Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. science, 2002. 297(5583): p. 1016–1018. [DOI] [PubMed] [Google Scholar]
- 21.Gibson DG, et al. , Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science, 2010. 329(5987): p. 52–56. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison CA, et al. , Design and synthesis of a minimal bacterial genome. Science, 2016. 351(6280). [DOI] [PubMed] [Google Scholar]
- 23.Tu Y, et al. , Mimicking the Cell: Bio-Inspired Functions of Supramolecular Assemblies. Chemical Reviews, 2016. 116(4): p. 2023–2078. [DOI] [PubMed] [Google Scholar]
- 24.York-Duran MJ, et al. , Recent advances in compartmentalized synthetic architectures as drug carriers, cell mimics and artificial organelles. Colloids and Surfaces B: Biointerfaces, 2017. 152: p. 199–213. [DOI] [PubMed] [Google Scholar]
- 25.Paleos CM, et al. , Formation of artificial multicompartment vesosome and dendrosome as prospected drug and gene delivery carriers. Journal of controlled release, 2013. 170(1): p. 141–152. [DOI] [PubMed] [Google Scholar]
- 26.Branco MC and Schneider JP, Self-assembling materials for therapeutic delivery. Acta biomaterialia, 2009. 5(3): p. 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amidi M, et al. , Antigen-expressing immunostimulatory liposomes as a genetically programmable synthetic vaccine. Systems and synthetic biology, 2011. 5(1–2): p. 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobed M and Chang T, Preparation and surface characterization of carboxymethylchitin-incorporated submicron bilayer-lipid membrane artificial cells (liposomes) encapsulating hemoglobin. Biomaterials, Artificial Cells and Immobilization Biotechnology, 1991. 19(4): p. 731–744. [DOI] [PubMed] [Google Scholar]
- 29.Buddingh BC and van Hest JCM, Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity. Accounts of chemical research, 2017. 50(4): p. 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noireaux V and Libchaber A, A vesicle bioreactor as a step toward an artificial cell assembly. Proceedings of the National Academy of Sciences of the United States of America, 2004. 101(51): p. 17669–17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douliez J-P, Perro A, and Béven L, Stabilization of All-in-Water Emulsions To Form Capsules as Artificial Cells. ChemBioChem, 2019. 20(20): p. 2546–2552. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande PP, Biswas S, and Torchilin VP, Current trends in the use of liposomes for tumor targeting. Nanomedicine, 2013. 8(9): p. 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg S, et al. , A review on Nano-therapeutic drug delivery carriers for effective wound treatment strategies. Asian Journal of Pharmacy and Pharmacology, 2018. 4(2): p. 90–101. [Google Scholar]
- 34.Silindir-Gunay M and Ozer AY, Liposomes and micelles as nanocarriers for diagnostic and imaging purposes, in Design of Nanostructures for Theranostics Applications. 2018, Elsevier. p. 305–340. [Google Scholar]
- 35.Jeong S, et al. , Toward Artificial Cells: Novel Advances in Energy Conversion and Cellular Motility. Advanced Functional Materials, 2020. 30(11): p. 1907182. [Google Scholar]
- 36.Rideau E, et al. , Liposomes and polymersomes: a comparative review towards cell mimicking. Chemical Society Reviews, 2018. 47(23). [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara K and Yanagisawa M, Generation of giant unilamellar liposomes containing biomacromolecules at physiological intracellular concentrations using hypertonic conditions. ACS synthetic biology, 2014. 3(12): p. 870–874. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H and Ogawa A, Preparation of a Millimeter-Sized Supergiant Liposome That Allows for Efficient, Eukaryotic Cell-Free Translation in the Interior by Spontaneous Emulsion Transfer. ACS Synthetic Biology, 2020. 9(7): p. 1608–1614. [DOI] [PubMed] [Google Scholar]
- 39.Stein H, et al. , Production of Isolated Giant Unilamellar Vesicles under High Salt Concentrations. Frontiers in Physiology, 2017. 8(63). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsumoto K, et al. , Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids and Surfaces B: Biointerfaces, 2009. 68(1): p. 98–105. [DOI] [PubMed] [Google Scholar]
- 41.Dimitrov D and Angelova M, Lipid swelling and liposome formation mediated by electric fields. Journal of electroanalytical chemistry and interfacial electrochemistry, 1988. 253(2): p. 323–336. [Google Scholar]
- 42.Shum HC, et al. , Double emulsion templated monodisperse phospholipid vesicles. Langmuir, 2008. 24(15): p. 7651–7653. [DOI] [PubMed] [Google Scholar]
- 43.Lombardo D, et al. , Colloidal stability of liposomes. AIMS Materials Science, 2019. 6(2): p. 200. [Google Scholar]
- 44.Li S-D and Huang L, Pharmacokinetics and Biodistribution of Nanoparticles. Molecular Pharmaceutics, 2008. 5(4): p. 496–504. [DOI] [PubMed] [Google Scholar]
- 45.NOUNOU M, et al. , In vitro drug release of hydrophilic and hydrophobic drug entities from liposomal dispersions and gels. Acta pharmaceutica, 2006. 56(3): p. 311–324. [PubMed] [Google Scholar]
- 46.Kiaie SH, et al. , Axial pharmaceutical properties of liposome in cancer therapy: Recent advances and perspectives. International Journal of Pharmaceutics, 2020. 581: p. 119269. [DOI] [PubMed] [Google Scholar]
- 47.Pauli G, Tang W-L, and Li S-D, Development and characterization of the solvent-assisted active loading technology (SALT) for liposomal loading of poorly water-soluble compounds. Pharmaceutics, 2019. 11(9): p. 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai F-C, Stuhrmann B.r., and Koenderink GH, Encapsulation of active cytoskeletal protein networks in cell-sized liposomes. Langmuir, 2011. 27(16): p. 10061–10071. [DOI] [PubMed] [Google Scholar]
- 49.Kurokawa C, et al. , DNA cytoskeleton for stabilizing artificial cells. Proceedings of the National Academy of Sciences, 2017. 114(28): p. 7228–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riaz MK, et al. , Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. International journal of molecular sciences, 2018. 19(1): p. 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, et al. , Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Progress in Polymer Science, 2017. 64: p. 1–22. [Google Scholar]
- 52.Hu X, et al. , Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules, 2017. 18(3): p. 649–673. [DOI] [PubMed] [Google Scholar]
- 53.Leong J, et al. , Engineering polymersomes for diagnostics and therapy. Advanced healthcare materials, 2018. 7(8): p. 1701276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsden HR, Gabrielli L, and Kros A, Rapid preparation of polymersomes by a water addition/solvent evaporation method. Polymer Chemistry, 2010. 1(9): p. 1512–1518. [Google Scholar]
- 55.Li S, et al. , Self-Assembled Poly (butadiene)-b-poly (ethylene oxide) Polymersomes as Paclitaxel Carriers. Biotechnology progress, 2007. 23(1): p. 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bermudez H, et al. , Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules, 2002. 35(21): p. 8203–8208. [Google Scholar]
- 57.Habel J, et al. , How preparation and modification parameters affect PB-PEO polymersome properties in aqueous solution. Journal of Polymer Science Part B: Polymer Physics, 2016. 54(16): p. 1581–1592. [Google Scholar]
- 58.Kunzler C, et al. , Giant Biodegradable Poly (ethylene glycol)-block-Poly (ε-caprolactone) Polymersomes by Electroformation. Macromolecular Bioscience, 2020: p. 2000014. [DOI] [PubMed] [Google Scholar]
- 59.Rijpkema SJ, et al. , Designing Molecular Building Blocks for Functional Polymersomes. Israel Journal of Chemistry, 2019. 59(10): p. 928–944. [Google Scholar]
- 60.Zhang S, et al. , Self-assembly of amphiphilic Janus dendrimers into uniform onion-like dendrimersomes with predictable size and number of bilayers. Proceedings of the National Academy of Sciences, 2014. 111(25): p. 9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nazemi A and Gillies ER, Dendrimersomes with photodegradable membranes for triggered release of hydrophilic and hydrophobic cargo. Chemical Communications, 2014. 50(76): p. 11122–11125. [DOI] [PubMed] [Google Scholar]
- 62.Hu F-F, et al. , Enthalpy-driven self-assembly of amphiphilic Janus dendrimers into onion-like vesicles: a Janus particle model. Nanoscale, 2019. 11(37): p. 17350–17356. [DOI] [PubMed] [Google Scholar]
- 63.Torre P, et al. , Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proceedings of the National Academy of Sciences, 2019. 116(31): p. 15378–15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arai N, Yasuoka K, and Zeng XC, Self-assembly of Janus oligomers into onion-like vesicles with layer-by-layer water discharging capability: A minimalist model. ACS nano, 2016. 10(8): p. 8026–8037. [DOI] [PubMed] [Google Scholar]
- 65.Jain K, et al. , IPN Dendrimers in Drug Delivery, in Interpenetrating Polymer Network: Biomedical Applications. 2020, Springer. p. 143–181. [Google Scholar]
- 66.Berkland C, et al. , Precise control of PLG microsphere size provides enhanced control of drug release rate. Journal of controlled release, 2002. 82(1): p. 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theberge AB, et al. , Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angewandte Chemie International Edition, 2010. 49(34): p. 5846–5868. [DOI] [PubMed] [Google Scholar]
- 68.Ramadan Q, et al. , Medical and industrial applications of microfluidic-based cell/tissue culture and organs-on-a-chip. Frontiers in bioengineering and biotechnology, 2019. 7: p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berthier J and Silberzan P, Microfluidics for biotechnology. 2010: Artech House. [Google Scholar]
- 70.Merrin J, Frontiers in Microfluidics, a Teaching Resource Review. Bioengineering (Basel, Switzerland), 2019. 6(4): p. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friddin MS, et al. , New Directions for Artificial Cells Using Prototyped Biosystems. Analytical Chemistry, 2019. 91(8): p. 4921–4928. [DOI] [PubMed] [Google Scholar]
- 72.Xie R, et al. , Engineering of Hydrogel Materials with Perfusable Microchannels for Building Vascularized Tissues. Small, 2020. 16(15): p. 1902838. [DOI] [PubMed] [Google Scholar]
- 73.Ai Y, et al. , Microfluidics for Biosynthesizing: from Droplets and Vesicles to Artificial Cells. Small, 2020. 16(9): p. 1903940. [DOI] [PubMed] [Google Scholar]
- 74.Thorsen T, et al. , Dynamic Pattern Formation in a Vesicle-Generating Microfluidic Device. Physical Review Letters, 2001. 86(18): p. 4163–4166. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Chan HF, and Leong KW, Advanced materials and processing for drug delivery: the past and the future. Advanced drug delivery reviews, 2013. 65(1): p. 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Hoog H-PM, Nallani M, and Tomczak N, Self-assembled architectures with multiple aqueous compartments. Soft Matter, 2012. 8(17): p. 4552–4561. [Google Scholar]
- 77.Malmstadt N, et al. , Automated Formation of Lipid-Bilayer Membranes in a Microfluidic Device. Nano Letters, 2006. 6(9): p. 1961–1965. [DOI] [PubMed] [Google Scholar]
- 78.Deng NN and Huck WT, Microfluidic formation of monodisperse coacervate organelles in liposomes. Angewandte Chemie International Edition, 2017. 56(33): p. 9736–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elani Y, et al. , Constructing vesicle-based artificial cells with embedded living cells as organelle-like modules. Scientific reports, 2018. 8(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Litschel T, et al. , Beating vesicles: encapsulated protein oscillations cause dynamic membrane deformations. Angewandte Chemie International Edition, 2018. 57(50): p. 16286–16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng H, et al. , Droplet-based microfluidics systems in biomedical applications. Electrophoresis, 2019. 40(11): p. 1580–1590. [DOI] [PubMed] [Google Scholar]
- 82.Mason AF, et al. , Hierarchical Self-Assembly of a Copolymer-Stabilized Coacervate Protocell. Journal of the American Chemical Society, 2017. 139(48): p. 17309–17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, et al. , Controllable microfluidic production of multicomponent multiple emulsions. Lab on a Chip, 2011. 11(9): p. 1587–1592. [DOI] [PubMed] [Google Scholar]
- 84.Lu AX, et al. , A new design for an artificial cell: polymer microcapsules with addressable inner compartments that can harbor biomolecules, colloids or microbial species. Chemical science, 2017. 8(10): p. 6893–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bayoumi M, et al. , Multi-compartment encapsulation of communicating droplets and droplet networks in hydrogel as a model for artificial cells. Scientific Reports, 2017. 7(1): p. 45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nuti N, Verboket PE, and Dittrich PS, Multivesicular droplets: a cell model system to study compartmentalised biochemical reactions. Lab on a Chip, 2017. 17(18): p. 3112–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elani Y, et al. , Constructing vesicle-based artificial cells with embedded living cells as organelle-like modules. Scientific Reports, 2018. 8(1): p. 4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim MW, et al. , A promising biocompatible platform: lipid-based and bio-inspired smart drug delivery systems for cancer therapy. International journal of molecular sciences, 2018. 19(12): p. 3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Q, Li X, and Zhao C, Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Frontiers in bioengineering and biotechnology, 2020. 8: p. 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matoori S and Leroux J-C, Twenty-five years of polymersomes: lost in translation? Materials Horizons, 2020. 7(5): p. 1297–1309. [Google Scholar]
- 91.Aibani N, Khan TN, and Callan B, Liposome mimicking polymersomes; A comparative study of the merits of polymersomes in terms of formulation and stability. International journal of pharmaceutics: X, 2020. 2: p. 100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yousefi M, Narmani A, and Jafari SM, Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Advances in colloid and interface science, 2020. 278: p. 102125. [DOI] [PubMed] [Google Scholar]
- 93.Dimitriou P, et al. , Droplet Microfluidics for Tumor Drug-Related Studies and Programmable Artificial Cells. Global Challenges, 2021: p. 2000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takinoue M and Takeuchi S, Droplet microfluidics for the study of artificial cells. Analytical and bioanalytical chemistry, 2011. 400(6): p. 1705–1716. [DOI] [PubMed] [Google Scholar]
- 95.Shanta V, et al. , Epidemiology of cancer of the cervix: global and national perspective. Journal of the Indian Medical association, 2000. 98(2): p. 49. [PubMed] [Google Scholar]
- 96.Hayat MJ, et al. , Age-Adjusted US Cancer Death Rate Predictions. Journal of data science : JDS, 2010. 8(2): p. 339–348. [PMC free article] [PubMed] [Google Scholar]
- 97.Golemis EA, et al. , Molecular mechanisms of the preventable causes of cancer in the United States. Genes & development, 2018. 32(13–14): p. 868–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Böhme D and Beck-Sickinger AG, Drug delivery and release systems for targeted tumor therapy. Journal of Peptide Science, 2015. 21(3): p. 186–200. [DOI] [PubMed] [Google Scholar]
- 99.Wang Z, et al. , Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. International journal of pharmaceutics, 2018. 535(1–2): p. 253–260. [DOI] [PubMed] [Google Scholar]
- 100.Chang TMS, Therapeutic applications of polymeric artificial cells. Nature Reviews Drug Discovery, 2005. 4(3): p. 221–235. [DOI] [PubMed] [Google Scholar]
- 101.Friedrich J, Ebner R, and Kunz-Schughart LA, Experimental anti-tumor therapy in 3-D: spheroids–old hat or new challenge? International journal of radiation biology, 2007. 83(11–12): p. 849–871. [DOI] [PubMed] [Google Scholar]
- 102.Schroeder A, et al. , Treating metastatic cancer with nanotechnology. Nature Reviews Cancer, 2012. 12(1): p. 39–50. [DOI] [PubMed] [Google Scholar]
- 103.Fenske DB and Cullis PR, Liposomal nanomedicines. Expert Opinion on Drug Delivery, 2008. 5(1): p. 25–44. [DOI] [PubMed] [Google Scholar]
- 104.Calixto G, et al. , Nanotechnology-based drug delivery systems for treatment of oral cancer: a review. International journal of nanomedicine, 2014. 9: p. 3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen H, et al. , Toxicity, pharmacokinetics, and in vivo efficacy of biotinylated chitosan surface-modified PLGA nanoparticles for tumor therapy. Artificial cells, nanomedicine, and biotechnology, 2017. 45(6): p. 1115–1122. [DOI] [PubMed] [Google Scholar]
- 106.Chang TMS, Artificial cells: biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation, and cell/stem cell therapy. Vol. 1. 2007: World Scientific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goh WJ, et al. , Doxorubicin-loaded cell-derived nanovesicles: an alternative targeted approach for anti-tumor therapy. International journal of nanomedicine, 2017. 12: p. 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carvalho FS, et al. , Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Medicinal research reviews, 2014. 34(1): p. 106–135. [DOI] [PubMed] [Google Scholar]
- 109.Abdolahpour S, et al. , Targeted delivery of doxorubicin into tumor cells by nanostructured lipid carriers conjugated to anti-EGFRvIII monoclonal antibody. Artificial cells, nanomedicine, and biotechnology, 2018. 46(1): p. 89–94. [DOI] [PubMed] [Google Scholar]
- 110.Barenholz YC, Doxil®—the first FDA-approved nano-drug: lessons learned. Journal of controlled release, 2012. 160(2): p. 117–134. [DOI] [PubMed] [Google Scholar]
- 111.Rafiyath SM, et al. , Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Experimental hematology & oncology, 2012. 1(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith JA, et al. , Is it equivalent? Evaluation of the clinical activity of single agent Lipodox® compared to single agent Doxil® in ovarian cancer treatment. Journal of Oncology Pharmacy Practice, 2015. 22(4): p. 599–604. [DOI] [PubMed] [Google Scholar]
- 113.Leo CP, et al. , FDA and EMA approvals of new breast cancer drugs—A comparative regulatory analysis. Cancers, 2020. 12(2): p. 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nadas J and Sun D, Anthracyclines as effective anticancer drugs. Expert Opinion on Drug Discovery, 2006. 1(6): p. 549–568. [DOI] [PubMed] [Google Scholar]
- 115.Xiong S, et al. , Preparation, therapeutic efficacy and intratumoral localization of targeted daunorubicin liposomes conjugating folate-PEG-CHEMS. Biomedicine & Pharmacotherapy, 2011. 65(1): p. 2–8. [DOI] [PubMed] [Google Scholar]
- 116.Creutzig U, et al. , Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood, 2013. 122(1): p. 37–43. [DOI] [PubMed] [Google Scholar]
- 117.Narvekar M, et al. , Nanocarrier for poorly water-soluble anticancer drugs—barriers of translation and solutions. Aaps Pharmscitech, 2014. 15(4): p. 822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang T, et al. , Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. International journal of Pharmaceutics, 2007. 338(1–2): p. 317–326. [DOI] [PubMed] [Google Scholar]
- 119.Zhang N, et al. , Gold conjugate-based liposomes with hybrid cluster bomb structure for liver cancer therapy. Biomaterials, 2016. 74: p. 280–291. [DOI] [PubMed] [Google Scholar]
- 120.Lu Y, et al. , Bioresponsive materials. Nature Reviews Materials, 2016. 2(1): p. 1–17. [Google Scholar]
- 121.Ashammakhi N and Kaarela O, Stimuli-responsive biomaterials: next wave. Journal of Craniofacial Surgery, 2017. 28(7): p. 1647–1648. [DOI] [PubMed] [Google Scholar]
- 122.Yatvin MB, et al. , Design of liposomes for enhanced local release of drugs by hyperthermia. Science, 1978. 202(4374): p. 1290–1293. [DOI] [PubMed] [Google Scholar]
- 123.Yarmolenko PS, et al. , Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. International Journal of Hyperthermia, 2010. 26(5): p. 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mao Y, et al. , Thermosensitive Hydrogel System With Paclitaxel Liposomes Used in Localized Drug Delivery System for In Situ Treatment of Tumor: Better Antitumor Efficacy and Lower Toxicity. Journal of Pharmaceutical Sciences, 2016. 105(1): p. 194–204. [DOI] [PubMed] [Google Scholar]
- 125.Mackanos MA, et al. , Laser-induced disruption of systemically administered liposomes for targeted drug delivery. Journal of biomedical optics, 2009. 14(4): p. 044009. [DOI] [PubMed] [Google Scholar]
- 126.Hossann M, et al. , Size of thermosensitive liposomes influences content release. Journal of Controlled Release, 2010. 147(3): p. 436–443. [DOI] [PubMed] [Google Scholar]
- 127.Hatzakis NS, et al. , How curved membranes recruit amphipathic helices and protein anchoring motifs. Nature chemical biology, 2009. 5(11): p. 835–841. [DOI] [PubMed] [Google Scholar]
- 128.Chang H-I and Yeh M-K, Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International journal of nanomedicine, 2012. 7: p. 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan F, et al. , Paclitaxel-liposome–microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. Journal of controlled release, 2013. 166(3): p. 246–255. [DOI] [PubMed] [Google Scholar]
- 130.Yoshizaki Y, et al. , Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials, 2014. 35(28): p. 8186–8196. [DOI] [PubMed] [Google Scholar]
- 131.Yuba E, et al. , Bleomycin-loaded pH-sensitive polymer–lipid-incorporated liposomes for cancer chemotherapy. Polymers, 2018. 10(1): p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bode C, et al. , Paclitaxel encapsulated in cationic liposomes: a new option for neovascular targeting for the treatment of prostate cancer. Oncology reports, 2009. 22(2): p. 321–326. [PubMed] [Google Scholar]
- 133.Jiang Y, et al. , Protein Toxin Chaperoned by LRP-1-Targeted Virus-Mimicking Vesicles Induces High-Efficiency Glioblastoma Therapy In Vivo. Advanced Materials, 2018. 30(30): p. 1800316. [DOI] [PubMed] [Google Scholar]
- 134.Gaspar MM, Perez-Soler R, and Cruz MEM, Biological characterization of L-asparaginase liposomal formulations. Cancer Chemotherapy and Pharmacology, 1996. 38(4): p. 373–377. [DOI] [PubMed] [Google Scholar]
- 135.Wang X, et al. , Galactose-decorated reduction-sensitive degradable chimaeric polymersomes as a multifunctional nanocarrier to efficiently chaperone apoptotic proteins into hepatoma cells. Biomacromolecules, 2013. 14(8): p. 2873–2882. [DOI] [PubMed] [Google Scholar]
- 136.Aydar E, Palmer CP, and Djamgoz MB, Sigma receptors and cancer: possible involvement of ion channels. Cancer research, 2004. 64(15): p. 5029–5035. [DOI] [PubMed] [Google Scholar]
- 137.Banerjee R, et al. , Anisamide-targeted stealth liposomes: A potent carrier for targeting doxorubicin to human prostate cancer cells. International Journal of Cancer, 2004. 112(4): p. 693–700. [DOI] [PubMed] [Google Scholar]
- 138.Lu L, et al. , Anisamide-decorated pH-sensitive degradable chimaeric polymersomes mediate potent and targeted protein delivery to lung cancer cells. Biomacromolecules, 2015. 16(6): p. 1726–1735. [DOI] [PubMed] [Google Scholar]
- 139.Iinuma H, et al. , Intracellular targeting therapy of cisplatin-encapsulated transferrin-polyethylene glycol liposome on peritoneal dissemination of gastric cancer. International Journal of Cancer, 2002. 99(1): p. 130–137. [DOI] [PubMed] [Google Scholar]
- 140.Tran TH, et al. , Microfluidic approach for highly efficient synthesis of heparin-based bioconjugates for drug delivery. Lab on a Chip, 2012. 12(3): p. 589–594. [DOI] [PubMed] [Google Scholar]
- 141.Cristiano MC, et al. , Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids and Surfaces B: Biointerfaces, 2017. 150: p. 408–416. [DOI] [PubMed] [Google Scholar]
- 142.Jiang Y, et al. , Protein toxin chaperoned by LRP-1-targeted virus-mimicking vesicles induces high-efficiency glioblastoma therapy in vivo. Advanced Materials, 2018. 30(30): p. 1800316. [DOI] [PubMed] [Google Scholar]
- 143.Belwal VK and Singh KP, Nanosilica-supported liposome (protocells) as a drug vehicle for cancer therapy. International journal of nanomedicine, 2018. 13(T-NANO 2014 Abstracts): p. 125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dagar S, et al. , VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: in vivo studies. Journal of Controlled Release, 2003. 91(1): p. 123–133. [DOI] [PubMed] [Google Scholar]
- 145.Igarashi A, et al. , Liposomal photofrin enhances therapeutic efficacy of photodynamic therapy against the human gastric cancer. Toxicology Letters, 2003. 145(2): p. 133–141. [DOI] [PubMed] [Google Scholar]
- 146.Bourré L, et al. , In vivo photosensitizing efficiency of a diphenylchlorin sensitizer: interest of a DMPC liposome formulation. Pharmacological Research, 2003. 47(3): p. 253–261. [DOI] [PubMed] [Google Scholar]
- 147.El-Aneed A, An overview of current delivery systems in cancer gene therapy. Journal of Controlled Release, 2004. 94(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 148.Tomanin R and Scarpa M, Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Current gene therapy, 2004. 4(4): p. 357–372. [DOI] [PubMed] [Google Scholar]
- 149.Sung YK and Kim SW, Recent advances in the development of gene delivery systems. Biomaterials Research, 2019. 23(1): p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaczmarek JC, Kowalski PS, and Anderson DG, Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome medicine, 2017. 9(1): p. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Litzinger DC and Huang L, Phosphatodylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applications. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes, 1992. 1113(2): p. 201–227. [DOI] [PubMed] [Google Scholar]
- 152.Wang C-Y and Huang L, pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proceedings of the National Academy of Sciences, 1987. 84(22): p. 7851–7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown MD, Schätzlein AG, and Uchegbu IF, Gene delivery with synthetic (non viral) carriers. International Journal of Pharmaceutics, 2001. 229(1–2): p. 1–21. [DOI] [PubMed] [Google Scholar]
- 154.Karmali PP and Chaudhuri A, Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Medicinal Research Reviews, 2007. 27(5): p. 696–722. [DOI] [PubMed] [Google Scholar]
- 155.Felgner PL, et al. , Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences, 1987. 84(21): p. 7413–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wiseman J, et al. , A comparison of linear and branched polyethylenimine (PEI) with DCChol/DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene therapy, 2003. 10(19): p. 1654–1662. [DOI] [PubMed] [Google Scholar]
- 157.Candiani G, et al. , Bioreducible liposomes for gene delivery: from the formulation to the mechanism of action. PLoS One, 2010. 5(10): p. e13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rayamajhi S, et al. , pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids and Surfaces B: Biointerfaces, 2020. 188: p. 110804. [DOI] [PubMed] [Google Scholar]
- 159.Ruponen M, Ylä-Herttuala S, and Urtti A, Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1999. 1415(2): p. 331–341. [DOI] [PubMed] [Google Scholar]
- 160.Bozzuto G and Molinari A, Liposomes as nanomedical devices. International journal of nanomedicine, 2015. 10: p. 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Matsuo T, et al. , Gene transfer to the retina of rat by liposome eye drops. Biochemical and biophysical research communications, 1996. 219(3): p. 947–950. [DOI] [PubMed] [Google Scholar]
- 162.Bozzuto G and Molinari A, Liposomes as nanomedical devices. International journal of nanomedicine, 2015. 10: p. 975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rytting E, et al. , Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert opinion on drug delivery, 2008. 5(6): p. 629–639. [DOI] [PubMed] [Google Scholar]
- 164.Koh CG, et al. , Delivery of polyethylenimine/DNA complexes assembled in a microfluidics device. Molecular Pharmaceutics, 2009. 6(5): p. 1333–1342. [DOI] [PubMed] [Google Scholar]
- 165.Lomas H, et al. , Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday discussions, 2008. 139: p. 143–159. [DOI] [PubMed] [Google Scholar]
- 166.Lomas H, et al. , Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery. Advanced Materials, 2007. 19(23): p. 4238–4243. [Google Scholar]
- 167.Kim Y, et al. , Polymersome delivery of siRNA and antisense oligonucleotides. Journal of Controlled Release, 2009. 134(2): p. 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh AP, et al. , Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal transduction and targeted therapy, 2019. 4(1): p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wilhelm S, et al. , Analysis of nanoparticle delivery to tumours. Nature reviews materials, 2016. 1(5): p. 1–12. [Google Scholar]
- 170.Raposo G and Stoorvogel W, Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology, 2013. 200(4): p. 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kanada M, Bachmann MH, and Contag CH, Signaling by extracellular vesicles advances cancer hallmarks. Trends in Cancer, 2016. 2(2): p. 84–94. [DOI] [PubMed] [Google Scholar]
- 172.Andaloussi SE, et al. , Extracellular vesicles: biology and emerging therapeutic opportunities. Nature reviews Drug discovery, 2013. 12(5): p. 347–357. [DOI] [PubMed] [Google Scholar]
- 173.Kanada M and Ashammakhi N, Extracellular vesicles: Emerging opportunities for tissue engineering and regenerative medicine. Journal of Craniofacial Surgery, 2021. [DOI] [PubMed] [Google Scholar]
- 174.Ural EE, et al. , Visualizing Extracellular Vesicles and Their Function in 3D Tumor Microenvironment Models. International Journal of Molecular Sciences, 2021. 22(9): p. 4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lenzini S, et al. , Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol, 2020. 15(3): p. 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liang Q, et al. , The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat Biomed Eng, 2019. 3(9): p. 729–740. [DOI] [PubMed] [Google Scholar]
- 177.Record M, et al. , Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2014. 1841(1): p. 108–120. [DOI] [PubMed] [Google Scholar]
- 178.Vader P, et al. , Extracellular vesicles for drug delivery. Advanced drug delivery reviews, 2016. 106: p. 148–156. [DOI] [PubMed] [Google Scholar]
- 179.Kamerkar S, et al. , Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 2017. 546(7659): p. 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yong T, et al. , Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nature communications, 2019. 10(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang X, et al. , Engineered Extracellular Vesicles for Cancer Therapy. Advanced Materials, 2020. [DOI] [PubMed] [Google Scholar]
- 182.Haney MJ, et al. , Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release, 2015. 207: p. 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Walker S, et al. , Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics, 2019. 9(26): p. 8001–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hoshino A, et al. , Tumour exosome integrins determine organotropic metastasis. Nature, 2015. 527(7578): p. 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rana S, et al. , Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. The international journal of biochemistry & cell biology, 2012. 44(9): p. 1574–1584. [DOI] [PubMed] [Google Scholar]
- 186.Laulagnier K, et al. , Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cellular and molecular life sciences, 2018. 75(4): p. 757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Piffoux M, et al. , Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS nano, 2018. 12(7): p. 6830–6842. [DOI] [PubMed] [Google Scholar]
- 188.Lin Y, et al. , Exosome–liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Advanced Science, 2018. 5(4): p. 1700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rayamajhi S, et al. , Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta biomaterialia, 2019. 94: p. 482–494. [DOI] [PubMed] [Google Scholar]
- 190.Kooijmans S, et al. , PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. Journal of Controlled Release, 2016. 224: p. 77–85. [DOI] [PubMed] [Google Scholar]
- 191.Murphy DE, et al. , Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Experimental & molecular medicine, 2019. 51(3): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luan X, et al. , Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica, 2017. 38(6): p. 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu M, et al. , Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. International journal of pharmaceutics, 2018. 550(1–2): p. 100–113. [DOI] [PubMed] [Google Scholar]
- 194.Saroja C, Lakshmi P, and Bhaskaran S, Recent trends in vaccine delivery systems: A review. International journal of pharmaceutical investigation, 2011. 1(2): p. 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marasini N, et al. , Chapter Twelve - Liposomes as a Vaccine Delivery System, in Micro and Nanotechnology in Vaccine Development, Skwarczynski M and Toth I, Editors. 2017, William Andrew Publishing. p. 221–239. [Google Scholar]
- 196.Amidi M, et al. , Antigen-expressing immunostimulatory liposomes as a genetically programmable synthetic vaccine. Systems and Synthetic Biology, 2011. 5(1): p. 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fan Y, et al. , Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. Journal of Controlled Release, 2015. 208: p. 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barnier Quer C, et al. , Cationic liposomes as adjuvants for influenza hemagglutinin: More than charge alone. European Journal of Pharmaceutics and Biopharmaceutics, 2012. 81(2): p. 294–302. [DOI] [PubMed] [Google Scholar]
- 199.Şenel S and Yüksel S, Chitosan-based particulate systems for drug and vaccine delivery in the treatment and prevention of neglected tropical diseases. Drug Delivery and Translational Research, 2020. [DOI] [PubMed] [Google Scholar]
- 200.Rescia VC, et al. , Dressing liposomal particles with chitosan and poly(vinylic alcohol) for oral vaccine delivery. Journal of Liposome Research, 2011. 21(1): p. 38–45. [DOI] [PubMed] [Google Scholar]
- 201.Cheng H-C, et al. , Effects of tremella–alginate–liposome encapsulation on oral delivery of inactivated H5N3 vaccine. Journal of Microencapsulation, 2011. 28(1): p. 55–61. [DOI] [PubMed] [Google Scholar]
- 202.Hamborg M, et al. , Elucidating the mechanisms of protein antigen adsorption to the CAF/NAF liposomal vaccine adjuvant systems: Effect of charge, fluidity and antigen-to-lipid ratio. Biochimica et Biophysica Acta (BBA) - Biomembranes, 2014. 1838(8): p. 2001–2010. [DOI] [PubMed] [Google Scholar]
- 203.Cole SL, et al. , Influenza vaccines using liposomal formulations of toll-like receptor (TLR) 7/8 and 4 agonists as adjuvants. The Journal of Immunology, 2020. 204(1 Supplement): p. 245.12–245.12. [Google Scholar]
- 204.Chen H, Torchilin V, and Langer R, Lectin-bearing Polymerized Liposomes as Potential Oral Vaccine Carriers. Pharmaceutical Research, 1996. 13(9): p. 1378–1383. [DOI] [PubMed] [Google Scholar]
- 205.Liau JJ, et al. , A lipid based multi-compartmental system: Liposomes-in-double emulsion for oral vaccine delivery. European Journal of Pharmaceutics and Biopharmaceutics, 2015. 97: p. 15–21. [DOI] [PubMed] [Google Scholar]
- 206.Heurtault B, Frisch B, and Pons F, Liposomes as delivery systems for nasal vaccination: strategies and outcomes. Expert Opinion on Drug Delivery, 2010. 7(7): p. 829–844. [DOI] [PubMed] [Google Scholar]
- 207.Wang C, et al. , Lymphatic-targeted cationic liposomes: A robust vaccine adjuvant for promoting long-term immunological memory. Vaccine, 2014. 32(42): p. 5475–5483. [DOI] [PubMed] [Google Scholar]
- 208.Wang N and Wang T, Preparation of Multifunctional Liposomes as a Stable Vaccine Delivery-Adjuvant System by Procedure of Emulsification-Lyophilization, in Vaccine Design: Methods and Protocols, Volume 2: Vaccines for Veterinary Diseases, Thomas S, Editor. 2016, Springer New York: New York, NY. p. 635–649. [DOI] [PubMed] [Google Scholar]
- 209.Khatri K, et al. , Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine, 2008. 26(18): p. 2225–2233. [DOI] [PubMed] [Google Scholar]
- 210.Lanza JS, et al. , A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. International Journal of Pharmaceutics, 2020. 586: p. 119390. [DOI] [PubMed] [Google Scholar]
- 211.Bookstaver ML, et al. , Improving vaccine and immunotherapy design using biomaterials. Trends in immunology, 2018. 39(2): p. 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wagner A and Vorauer-Uhl K, Liposome Technology for Industrial Purposes. Journal of Drug Delivery, 2011. 2011: p. 591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Phillips NC and Gagné L, Modulation of Murine Macrophage Nitric Oxide Synthesis by Liposomal Phospholipids: Correlation with Liposome Immune Adjuvant Activity. Journal of Drug Targeting, 1995. 3(2): p. 137–147. [DOI] [PubMed] [Google Scholar]
- 214.Phillips NC, et al. , Influence of phospholipid composition on antibody responses to liposome encapsulated protein and peptide antigens. Vaccine, 1996. 14(9): p. 898–904. [DOI] [PubMed] [Google Scholar]
- 215.Kaur R, et al. , Effect of Incorporating Cholesterol into DDA:TDB Liposomal Adjuvants on Bilayer Properties, Biodistribution, and Immune Responses. Molecular Pharmaceutics, 2014. 11(1): p. 197–207. [DOI] [PubMed] [Google Scholar]
- 216.Nakano Y, et al. , Cholesterol inclusion in liposomes affects induction of antigen-specific IgG and IgE antibody production in mice by a surface-linked liposomal antigen. Bioconjug Chem, 2002. 13(4): p. 744–9. [DOI] [PubMed] [Google Scholar]
- 217.Boyer C and Zasadzinski JA, Multiple Lipid Compartments Slow Vesicle Contents Release in Lipases and Serum. ACS Nano, 2007. 1(3): p. 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vrieze J.d. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. 2021; Available from: https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions.
- 219.Ertas YN, et al. , Role of biomaterials in the diagnosis, prevention, treatment, and study of corona virus disease 2019 (COVID-19). Emergent Materials, 2021: p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McKinlay CJ, et al. , Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proceedings of the National Academy of Sciences, 2017. 114(4): p. E448–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Barnier Quer C, et al. , Polymersomes enhance the immunogenicity of influenza subunit vaccine. Polymer Chemistry, 2011. 2(7): p. 1482–1485. [Google Scholar]
- 222.Galan-Navarro C, et al. , Oxidation-sensitive polymersomes as vaccine nanocarriers enhance humoral responses against Lassa virus envelope glycoprotein. Virology, 2017. 512: p. 161–171. [DOI] [PubMed] [Google Scholar]
- 223.Wibowo D, et al. , Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials, 2021. 268: p. 120597–120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tizard IR, Adjuvants and adjuvanticity. Vaccines for Veterinarians, 2021: p. 75. [Google Scholar]
- 225.Machhi J, et al. , Nanocarrier vaccines for SARS-CoV-2. Advanced Drug Delivery Reviews, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang N, Chen M, and Wang T, Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. Journal of Controlled Release, 2019. 303: p. 130–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Perrie Y, et al. , Designing liposomal adjuvants for the next generation of vaccines. Advanced drug delivery reviews, 2016. 99: p. 85–96. [DOI] [PubMed] [Google Scholar]
- 228.Boeckler C, et al. , Design of highly immunogenic liposomal constructs combining structurally independent B cell and T helper cell peptide epitopes. European Journal of Immunology, 1999. 29(7): p. 2297–2308. [DOI] [PubMed] [Google Scholar]
- 229.Roth A, et al. , Induction of effective and antigen-specific antitumour immunity by a liposomal ErbB2/HER2 peptide-based vaccination construct. British Journal of Cancer, 2005. 92(8): p. 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jalali SA, et al. , Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD Nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2012. 8(5): p. 692–701. [DOI] [PubMed] [Google Scholar]
- 231.Abasian P, et al. , Polymeric nanocarriers in targeted drug delivery systems: A review. Polymers for Advanced Technologies, 2020. 31(12): p. 2939–2954. [Google Scholar]
- 232.Filatov N, et al. Comparison of step and flow-focusing emulsification methods for water-in-oil monodisperse drops in microfluidic chips. in Journal of Physics: Conference Series. 2018. IOP Publishing. [Google Scholar]
- 233.Toparlak ÖD, et al. , Artificial cells drive neural differentiation. Science advances, 2020. 6(38): p. eabb4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lentini R, Martín NY, and Mansy SS, Communicating artificial cells. Current opinion in chemical biology, 2016. 34: p. 53–61. [DOI] [PubMed] [Google Scholar]
- 235.Anselmo AC, et al. , Exploiting shape, cellular-hitchhiking and antibodies to target nanoparticles to lung endothelium: Synergy between physical, chemical and biological approaches. Biomaterials, 2015. 68: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 236.Brenner JS, Mitragotri S, and Muzykantov VR, Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annual Review of Biomedical Engineering, 2021. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Krishnamachari Y, Pearce ME, and Salem AK, Self-Assembly of Cell–Microparticle Hybrids. Advanced materials, 2008. 20(5): p. 989–993. [Google Scholar]
- 238.Ahmed KK, Geary SM, and Salem AK, Surface engineering tumor cells with adjuvant-loaded particles for use as cancer vaccines. Journal of Controlled Release, 2017. 248: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]