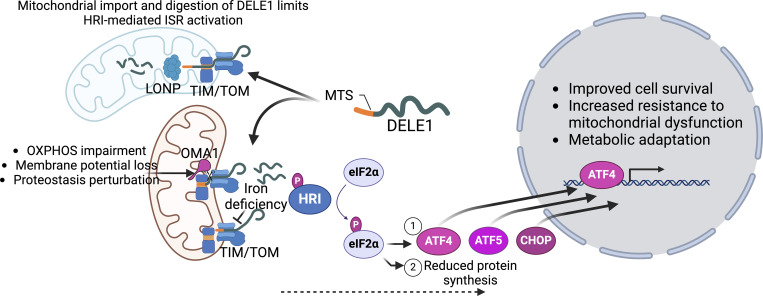
Figure 2.
Regulation of the ISR during mitochondrial stress. The protein DELE1 harbors an amino-terminal MTS and is constitutively imported into functional mitochondria where it is degraded by the protease LONP. However, during mitochondrial stress caused by OXPHOS perturbations or depletion of the mitochondrial inner membrane potential, DELE1 import is stalled with the C-terminal domain remaining in the cytosol. Iron deficiency causes DELE1 to remain in the TOM channel, while inner membrane uncoupling causes OMA1 to cleave DELE1 allowing the C-terminal fragment to diffuse back into the cytosol. Cytosolic DELE1 oligomerizes, binds, and activates the ISR kinase HRI. Subsequent phosphorylation of eIF2α (1) reduces the rate of total protein synthesis while (2) increasing the translation of three transcription factors (ATF4, CHOP, and ATF5) that promote survival during mitochondrial dysfunction.