Figure 2. Time to Resolution of 5 COVID-19 Symptoms in the Intention-to-Treat Population.
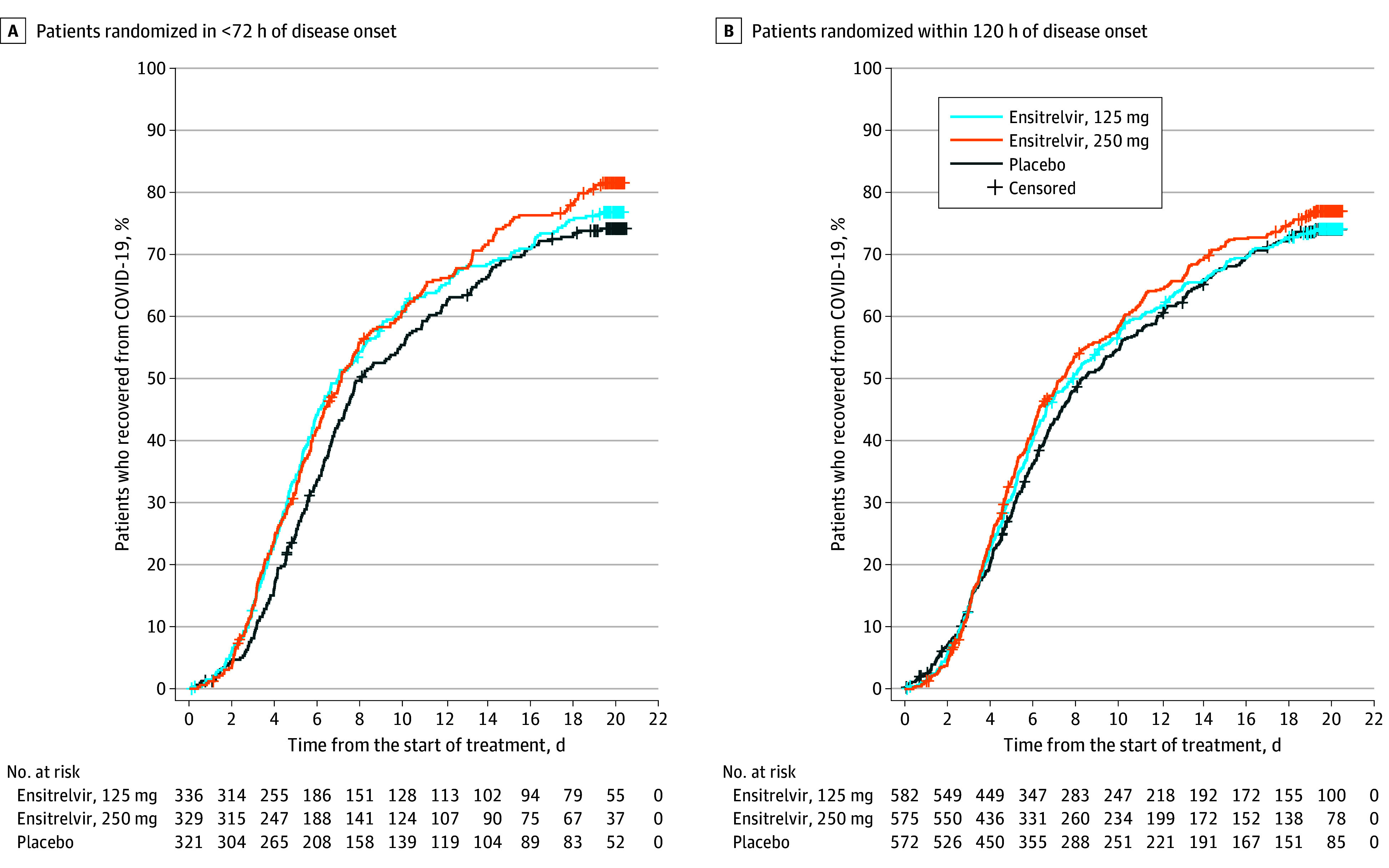
The analysis was performed for all patients who tested positive for SARS-CoV-2 RNA at baseline. Patients randomized in less than 72 hours of disease onset in the 125-mg ensitrelvir group were defined as the primary analysis population. A Peto-Prentice generalized Wilcoxon test was applied to test the statistical significance vs placebo. The test was stratified by SARS-CoV-2 vaccination history (yes or no) for patients randomized in less than 72 hours (A) and time from onset to randomization (<72 or ≥72 hours) and SARS-CoV-2 vaccination history (yes or no) for patients randomized within 120 hours (B). The 5 COVID-19 symptoms were stuffy or runny nose, sore throat, cough, feeling hot or feverish, and low energy or tiredness. In the primary analysis population (A), the median time to resolution of the 5 COVID-19 symptoms was 171.2 hours in the 250-mgensitrelvir group and 192.2 hours in the placebo group (difference, −21.0 hours; 95% CI, −73.8 to 7.2 hours). Among patients randomized within 120 hours of disease onset (B), the median difference in the time to resolution of the 5 COVID-19 symptoms between 125-mg ensitrelvir and placebo was −10.6 hours (95% CI, −56.9 to 21.3 hours).
