Abstract
The dura mater, as the crucial outermost protective layer of the meninges, plays a vital role in safeguarding the underlying brain tissue. Neurosurgeons face significant challenges in dealing with trauma or large defects in the dura mater, as they must address the potential complications, such as wound infections, pseudomeningocele formation, cerebrospinal fluid leakage, and cerebral herniation. Therefore, the development of dural substitutes for repairing or reconstructing the damaged dura mater holds clinical significance. In this review we highlight the progress in the development of dural substitutes, encompassing autologous, allogeneic, and xenogeneic replacements, as well as the polymeric-based dural substitutes fabricated through various scaffolding techniques. In particular, we explore the development of composite materials that exhibit improved physical and biological properties for advanced dural substitutes. Furthermore, we address the challenges and prospects associated with developing clinically relevant alternatives to the dura mater.
Keywords: Dura mater, dural substitutes, brain trauma and injury, polymeric scaffolds, composites, tissue engineering
Graphical abstract.
Introduction
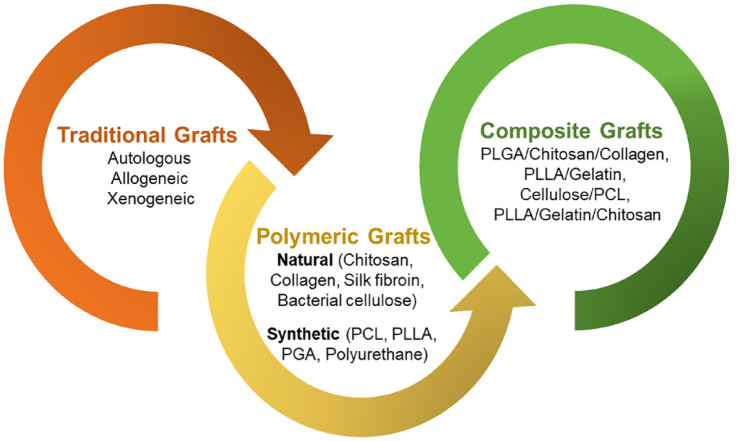
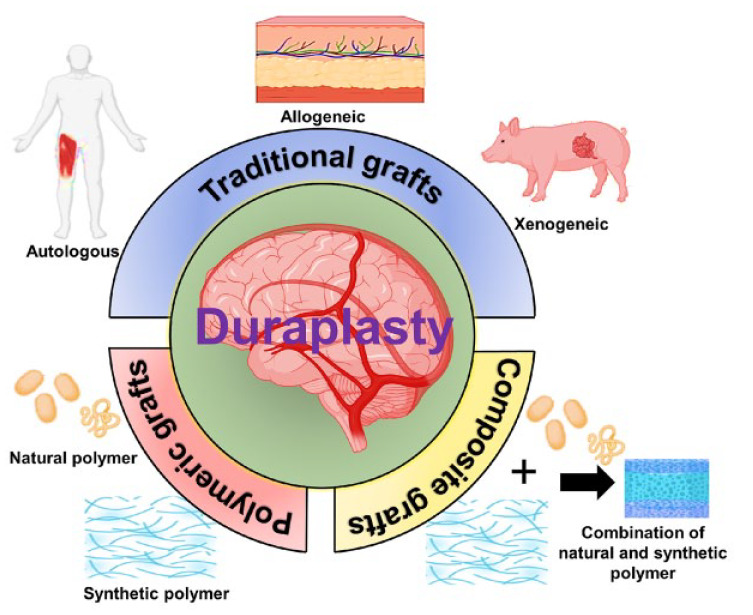
The dura mater is a significant protective membrane layer that is part of the central nervous system’s anatomy. Its integrity is crucial for safeguarding the brain tissue and sustaining neuro-electrical processes since it serves as a natural barrier.1,2 When dural defects occur due to trauma, tumor invasion, cranial/spinal surgeries, or clinical procedures, they can lead to various complications such as seizures, meningitis, cerebrospinal fluid leakage, cerebral herniation, pseudomeningocele, and infection. 3 While minor dura mater defects or damages can be repaired with sutures, substantial damages resulting from cranio-cerebral trauma, tumor invasion, or rupture necessitate the expansion or replacement of the damaged dura mater with substitutes. 4 Dural replacements have proven effective in restoring the water-tightness of the dura mater and preventing the affected area of the brain or spinal cord from impacting nearby tissues.5,6 Figure 1 illustrates the conventional substitutes commonly used for dural replacement, including autologous, allogeneic, and xenogeneic grafts.
Figure 1.
Illustrates a schematic representation of the dural substitutes currently employed for dural repair.
Autologous materials such as Fascia lata and Pericranium are commonly utilized by neurosurgeons as dural substitutes due to their non-toxic, vascularized, and nonimmunogenic nature. However, they have limitations, including limited supply and unsuitability for repairing large dural defects. Moreover, pericranium has the potential to be an ideal source for dural substitutes as it eliminates the need for additional surgery at the donor site and can be obtained during craniotomy. However, due to its thin and fragile nature, pericranium presents certain limitations in terms of surgical handling and suturing.7,8 Allogeneic dural substitutes, such as amniotic membranes, human acellular dermis, and small intestine submucosa, face issues including limited material sources, immune reactions, scarring, and encapsulation. 4 Xenogeneic materials, like porcine pericardium, carry a very high risk of disease transmission.9,10 An ideal dural replacement material would effectively repair and restore dural integrity at the defect site while possessing optimal biological and mechanical qualities comparable to or superior to autologous dura mater. It would provide a suitable framework for autologous healing, promoting fibroblast migration, attachment, and matrix resorption for structural or functional repair. Additionally, the ideal dural substitute should be biocompatible, promote tissue growth without adherence to surrounding tissues, prevent cerebrospinal fluid leakage (CSF), and inhibit scarring.11 –13 In recent decades, various artificial materials have been investigated as potential solutions to overcome the limitations of conventional substitutes. These materials offer a promising approach for reconstructing damaged or diseased dura mater, as they are readily available, cost-effective, and can be processed to mimic the physicochemical, mechanical, and biological properties of the dural membrane.
Both natural and synthetic biopolymers have been extensively investigated for the development of suitable dural substitutes. Natural biopolymers, including collagen, silk fibroin, chitosan, and bacterial cellulose, as well as synthetic biopolymers such as polycaprolactone (PCL), polyglycolic acid (PGA), polyurethane, and poly (L-lactic acid) (PLLA), have been introduced as polymeric materials to meet the requirements of an ideal dural substitute, as depicted in Figure 1. However, natural polymers have the drawback of having limited control over the degradation of the graft whereas synthetic polymers have the disadvantage of possessing weak cell affinity.14 –17 Therefore, researchers have realized the benefits of utilizing a combination of natural and synthetic polymers to develop dural substitutes that exhibit controlled biodegradability along with desired physicochemical, mechanical, and biological properties. Composite materials, which combine natural and synthetic polymeric materials, have been introduced to address these limitations. Although clinical exploration of composite alternatives for dural repair is still in its early stages, composite materials such as collagen/PLGA/chitosan and PLA/PCL/collagen have shown promising results, meeting the criteria of an ideal dural substitute. These dural replacements have been developed to possess favorable mechanical and biochemical qualities, and animal models have shown no signs of foreign body reactions.18 –20
In this review, we provide a comprehensive examination of the anatomical structure and composition of the human dura mater, encompassing both the cranial and spinal dura mater and its repair mechanism. We also explore the advancements in dural substitutes available for dura mater repair, as depicted in Figure 2. Furthermore, we shed light on the traditional grafts utilized in dura mater repair, including autologous, allogeneic, and xenogeneic substitutes, while discussing their respective advantages and disadvantages. Additionally, we discuss the clinical findings associated with the different types of polymeric dural substitutes, both natural and synthetic, developed between 2005 and 2023. The emerging utilization of composite materials for dura mater substitutes is also emphasized. The effectiveness of dural sealants in providing water-tight closure to prevent dural leakage has also been highlighted. Furthermore, we summarize the key properties of different dural substitutes, including their benefits, drawbacks, mechanical properties, conducted studies, and anti-cerebrospinal fluid (CSF) leakage properties. Finally, we address the challenges and future prospects in the development of dura mater substitutes.
Figure 2.
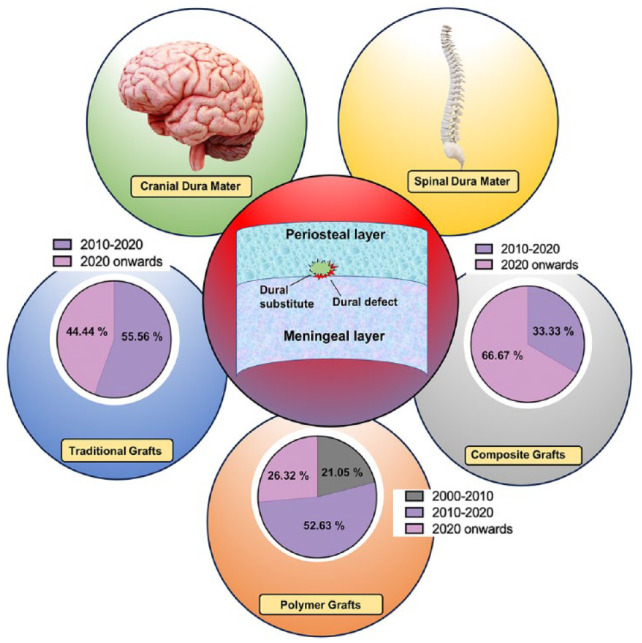
Schematic representation showing the dural defect and the available substitutes for dura mater repair. It also presents the relative percentage of studies conducted on dural grafts categorized by year.1,10,11,16,18,20 –47
Structure of the human dura mater
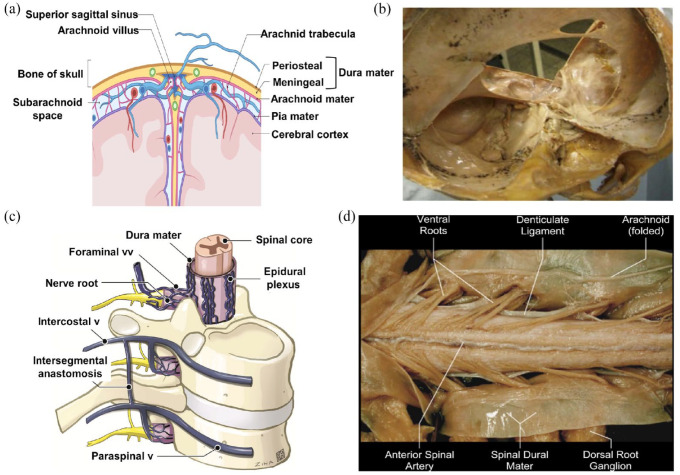
The human dura mater has a layered structure consisting of two main layers: the periosteal layer and the meningeal layer. The periosteal layer is the outermost layer and is in direct contact with the inner surface of the skull. It is composed of dense connective tissue and is highly vascularized. The meningeal layer is located beneath the periosteal layer and is closely associated with the brain and spinal cord. It consists of fibrous connective tissue and is responsible for providing protection and support to the central nervous system. Figure 3(a) depicts the three consecutive layers that make up the meninges: the dura mater, arachnoid, and pia mater. These layers serve as protective coverings for the brain and spinal cord. The pia mater and arachnoid are collectively referred to as the leptomeninx, representing the thinner meningeal layers, while the dura mater is known as the pachymeninx due to its thicker and more fibrous nature.48,49 The outermost layer of the meninges is the dura mater, which is fibrous and opaque. It plays a crucial role in safeguarding the brain and spinal cord and preventing the leakage of cerebrospinal fluid (CSF). In the spinal cord, it is referred to as the spinal dura mater, while in the skull, it is known as the cranial dura mater.
Figure 3.
Detailed representation of the dura mater present in the cranium and spine: (a) a diagram showing the meninges of the human head which includes the dura mater, the arachnoid mater and the pia, (b) a close-up view of the cranial dura mater. 50 Copyright 2021, Scientific Research Publishing, 50 (c) a diagram of the spinal cord showing the dura mater as a protective covering. 52 Copyright 2022, American Society of Neuroradiology. 52 (d) A close-up view of the cranial dura mater. 51 Copyright 2021, Scientific Research Publishing. 51
The dura mater is composed of two layers: the outer layer, known as the endosteal or periosteal layer, and the thicker inner layer called the meningeal layer. The endosteal layer of the dura mater forms the periosteum on the inner side of the skull. 53 The average thickness of the dura mater has been estimated to be 1.06 ± 0.22 mm 50 Despite anatomical differences between the cranial and spinal dura mater, both layers are impermeable to cerebrospinal fluid. The spinal dura mater is a tubular sheath surrounding the spinal cord, consisting of inelastic collagen fibers, as depicted in Figure 3(c) and (d). The transition from the cranial dura mater to the spinal dura mater occurs at the foramen magnum, where the outer periosteal layer terminates, and the inner meningeal layer forms the true spinal dura mater. 51 The average thickness of the spinal dura mater is approximately 1.106 ± 0.244 mm. 54 These details highlight the intricate structure and composition of the dura mater, both in the cranial and spinal regions, emphasizing their protective functions and differences in thickness.
Figure 3(b) illustrates how the cranial dura mater lines the interior of the skull, providing protection to the underlying structures. The cranial dura mater is composed of two outermost layers: the endosteal layer and the periosteal layer. These layers together form the cranial dura mater. The inner meningeal layer of the cranial dura mater is in direct contact with the dural border cells, while the endosteal layer adheres to the inner surface of the skull bones. 51 This arrangement helps secure the dura mater in place and maintain its structural integrity. The average thickness of the cranial dura mater has been estimated to be approximately 564 ± 50 μm. 55 This thickness may vary slightly among individuals. Understanding the thickness of the cranial dura mater is important in surgical procedures or when considering the use of dural substitutes. The cranial dura mater possesses specific mechanical properties. Its ultimate tensile strength, which measures the maximum stress it can withstand before breaking, is approximately 7.2 ± 0.4 MPa. The elastic modulus, which indicates the stiffness of the material, is around 61.7 MPa. These mechanical properties contribute to the dura mater’s ability to withstand external forces and maintain the integrity of the cranial cavity. These explanations provide a deeper understanding of the structure and characteristics of the cranial dura mater, emphasizing its composition, thickness, and mechanical properties.
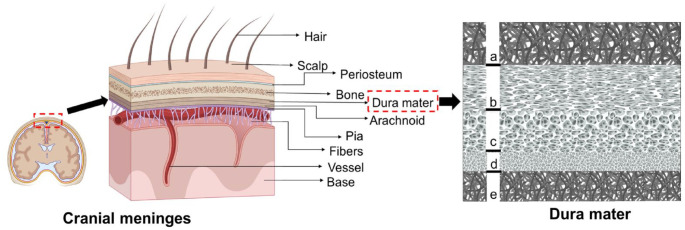
The composition of the dura mater primarily consists of collagen, elastin, fibroblast cells, and a few osteoblasts. Osteoblast cells are mainly found in the periosteum, which is a component of the dura mater. 50 A study conducted by Protasoni et al. 56 utilized scanning electron microscopy (SEM) to analyze the fibrous-porous structure of the human dura mater in detail (Figure 4). Based on their analysis, the human dura mater can be divided into five layers. The outermost layer is the bone surface layer (a), followed by the fibrous dura layer, which is further subdivided into the external median layer (b), the vascular layer (c), and the internal median layer (d). The innermost layer is the arachnoid layer (e), which is in contact with the dural border cells. The periosteal layer of the cranial dura mater consists of elongated and flattened cells that resemble fibroblasts. This layer also contains a few osteocytes, nerve fibers, and blood vessels. In comparison, the meningeal layer has a higher concentration of fibroblast cells and a lower amount of extracellular matrix. 57 The dural border cell layer is a distinct morphological layer consisting of flattened fibroblasts. 48 This layer lacks extracellular collagen but exhibits significant extracellular gaps. This layer has been referred to in certain studies as the dural mesothelium or neurothelium, and it has been suggested that it acts as a barrier to material diffusion across the dura’s inner surface. On the other hand, the cells of this layer seemed to resemble the flattened fibroblasts that are also present throughout the dura mater. It appears unlikely that this layer would serve as a very strong resistance to diffusion through the dura’s surface due to the lack of distinct morphological characteristics and tight connections between these cells. Thus, its specific function and role need to be further studied. 58
Figure 4.
A schematic representation of the cranial meninges and transverse section of the dura mater as seen in the SEM image where (a) is the bone surface layer, (b) represents the external median layer, (c) is the vascular layer, (d) is internal median layer, and (e) represents the arachnoid layer. 56
Elastic fibers within the dura mater occupy approximately 1.376 ± 1.766% of the total area and are randomly oriented with respect to collagen fibers. The outer layer of the dura mater contains a higher proportion of elastic fibers compared to the inner layer. 59 These additional explanations provide a more comprehensive understanding of the composition and structure of the dura mater, highlighting the presence of collagen, elastin, fibroblast cells, osteoblasts, and different layers within the dura mater.
The dural repair mechanism
The development of connective tissue fibers and fibroblastic proliferation, which are triggered by the dural edge or surrounding tissues, are the primary causes of regeneration in dural defects. Larger dural defects require the use of dural substitutes, which can mimic the tissue’s natural structure and keep the defect’s severity from spreading to neighboring tissues whereas the smaller dural defects can be fixed with sutures. The regeneration of the dura mater is correlated with the graft degradation. The introduced dural substitute acts as a framework facilitating the migration and integration of different cell types that produce collagen fibers, elastic fibers and glycoproteins such as fibroblasts, macrophages and lymphocytes during the dural reconstruction phase. Concurrently, fibroblasts and other cells release matrix metalloproteinases (MMPs) which can break down the implanted collagen-based dural substitute, aided by certain non-specific enzymes.4,60
Laun et al. 61 reported the use of bovine pericardium as a dural substitute. After 4 days of the dural implant, it showed migration of histocytes and fibroblasts into the implant where these cells used the natural pores of the graft and moved to the spaces available. It was observed that there was good vascularization present in the region between the implanted dural replacements and the new tissue. 61 Also, the chemical composition and thickness of the material, the processing method used for preparation, the graft’s location, and other variables affect the degradability of the dural substitute. For instance, Collagen type-I the main component present in the dural substitutes composed of two α1 chains and one α2 chain. Collagen is primarily broken into fragments by MMP-mediated collagen degradation, which predominantly occurs at the location on the α2 chain. As a result, several different non-specific proteases break down these two fragments into oligopeptides or amino acids. 62 These insights contribute to our understanding of effective strategies for dural regeneration and provide a foundation for further advancements in this field.
Conventional grafts for dural closure
Traditional grafts commonly used for dura mater repair include autologous, allogeneic, and xenogeneic grafts, as summarized in Table 1. The primary advantage of autologous grafts in dural repair is their ability to avoid immunogenic responses or adverse host reactions, whereas allogeneic and xenogeneic grafts offer surgical convenience as well as easy access during duraplasty. Due to these features and their affordability, conventional grafts are a suitable dural replacement for neurosurgeons.
Table 1.
Summarized data of the conventional dural substitutes with their advantages, limitations, study type, no. of subjects, mechanical properties, and anti-CSF leakage properties.
| Type of Dural substitute | Advantages | Limitations | Type of study | No. of subjects | Utimate tensile strength (MPa) | Young’s modulus (MPa) | CSF leakage | References |
|---|---|---|---|---|---|---|---|---|
| Fascia lata | No relapse of surgical site infection Less chances of death |
Sample size is too small | Retrospective study | 19 | NA | NA | NA | Zeng et al. 21 |
| Pericranium | No wound complications | Long incision required to harvest | Retrospective study | 51 | NA | NA | 9% | Hoffman et al. 22 |
| Amniotic membrane | No cerebral cortex adhesion and good integrative ability with the native dura | Sample size is too small and amniotic membrane is expensive | Clinical study | 25 | NA | NA | Observed in one patient | Marton et al. 23 |
| Amniotic membrane | No adverse reactions | NA | Retrospective feasibility study | 120 | NA | NA | Observed in two patients | Eichberg et al. 24 |
| Acellular human dermis | Immunologically inert, provides watertight closure and prevents adhesion to surrounding tissues | NA | Case report | 1 | NA | NA | Not observed | Lee et al. 25 |
| Equine pericardium membrane (heart → membrane) | Watertight closure, no disease transmission | Short follow-up time | Short-term investigation | 8 | NA | NA | Not observed | Centonze et al. 26 |
| Porcine pericardium | Transparency helps in preventing subdural hemorrhage after duraplasty | Sample size small and short follow-up time | In vivo study (pigs) | 6 | NA | NA | Not observed | Seo et al. 27 |
| Porcine small intestinal submucosa (Biodesign → ) | Better integration with the native dura mater | Sample size small and short follow-up time | In vivo study (pigs) | 6 | NA | NA | Not observed | Seo et al. 27 |
| Porcine peritoneal acellular matrix | Good anti-adhesion property | NA | In vitro and In vivo study | NA | NA | NA | NA | Yu et al. 1 |
| Acellular swim bladder | No adhesion observed to cerebral cortex | Slight inflammation observed | In vitro and in vivo study (rabbits) | NA | NA | NA | Not observed | Li et al. 10 |
Autologous dural grafts
Autologous dural grafts, such as fascia lata and pericranium, offer several advantages over other types of grafts. They are non-immunogenic and non-toxic, leading to minimal inflammatory or allergic reactions. Additionally, they provide effective watertight closure, ensuring the prevention of cerebrospinal fluid (CSF) leakage, and they are cost-effective. Fascia lata, although an efficient autologous dural graft, requires an additional surgical procedure to harvest it. On the other hand, pericranium, another commonly used autograft, eliminates the need for an extra incision since it can be obtained during craniotomy. 63 Recent studies have shown the effectiveness of fascia lata as a dural substitute in specific cases. For example, Zeng et al. 21 reported successful use of fascia lata in reconstructing the dura mater for patients with surgical site infections caused by multidrug-resistant (MDR) gram-negative bacteria following postcraniotomy procedures. The findings demonstrated that fascia lata was an efficient and feasible alternative, resulting in no relapse of surgical site infection and reduced odds of death. In contrast, the use of other artificial dural substitutes often led to intracranial infections caused by MDR gram-negative bacteria. 21
Pericranium is the second most commonly chosen autograft for dural replacement or repair surgery. In a study by Hoffman et al. 22 pericranium was compared to alloderm (allograft from human cadaver dermal matrix) for duraplasty in patients with Type I Chiari malformation. The findings showed that pericranium had fewer cases of CSF leakage and no wound complications compared to alloderm. However, it should be noted that pericranium requires a longer incision for its use as a dural substitute. Additionally, no wound complications were associated with pericranium, although it does require a longer incision for usage as a dural substitute. 22 While autologous grafts like pericranium and fascia lata have advantages such as easy availability, low cost, and absence of disease transmission risk, they do come with some drawbacks. These include the need for additional surgical incisions for graft harvesting, potential morbidity, postoperative discomfort, infection, reduced cosmesis, or local tissue injury. To address these limitations, allografts have gained acceptance as an alternative for dura mater repair.64 –66 Allogeneic grafts offer a readily available supply without the need for additional surgeries. They overcome the limitations associated with autologous grafts; however, they may present challenges related to immune reactions, scarring, and encapsulation. In summary, the use of autologous grafts such as pericranium and fascia lata has proven effective for dural repair, but they have associated disadvantages requiring additional incisions and potential morbidity. Allografts have emerged as an alternative option to overcome these limitations, providing a readily available supply while still posing some challenges.
Allogeneic dural grafts
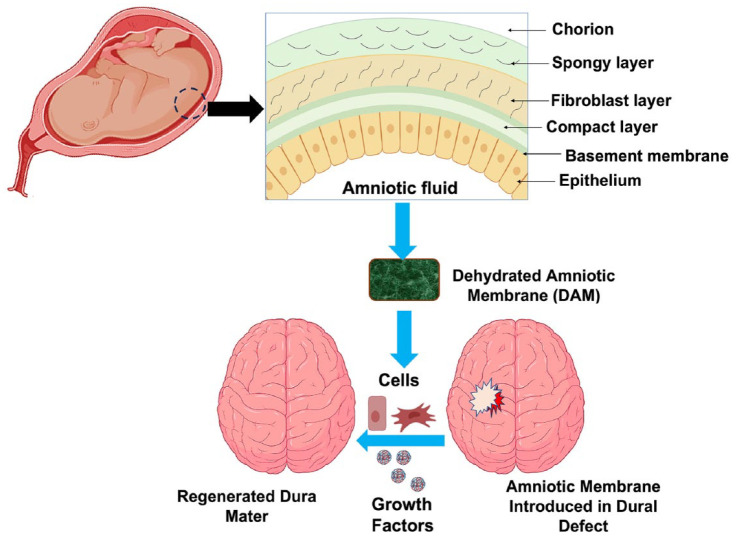
Allografts, such as acellular human dermis and amniotic membranes, are commonly used as substitutes for the dura mater.25,67 The acellular human dermis and amniotic membranes offer several advantages as allograft options because acellular grafts are non-immunogenic and can impart native biophysical and biochemical cues that support regeneration.68,69 A clinical study conducted by Marton et al. 23 focused on the usage of amniotic membranes as an allograft substitute for the dura mater in decompressive craniectomy procedures. Amniotic membranes possess anti-inflammatory properties that reduce the risk of scar formation, and they also promote wound healing by enhancing cell recruitment and differentiation. The lyophilized human amniotic membrane has been found to provide efficient water-tight closure, preventing CSF leakage at the dural defect site. Moreover, the amniotic membrane integrates well with the original dura mater, reducing the rates of death and infection among patients. 23
Eicheberg et al. 24 also reported on the application of an amniotic membrane as a substitute for the dura mater. Their study focused on the use of dehydrated amniotic membranes for repairing dura mater in transsphenoidal endoscopic endonasal surgery (TEES), as depicted in Figure 5. TEES requires a substitute for the dural defect that provides complete water-tight closure, ensuring clear separation between the intracranial and extradural compartments. The dehydrated amniotic membrane used in TEES demonstrated effective water-tight closure, preventing CSF leakage, and showed no adverse reactions. Additionally, the amniotic membrane contains growth factors that aid in cell differentiation, proliferation, and migration. Therefore, the amniotic membrane is considered a suitable alternative to autografts for dura mater reconstruction or repair. 24 In summary, allograft options like acellular human dermis and amniotic membranes offer benefits such as anti-inflammatory properties, wound healing capabilities, efficient water-tight closure, and integration with the original dura mater. These qualities make them viable substitutes for autografts in dura mater reconstruction or repair procedures.
Figure 5.
A schematic overview of the preparation, implantation, and regeneration process of the dehydarated amniotic membrane: illustrative depiction of the amniotic membrane as a viable substitute for autografts in the context of dura mater reconstruction or repair. 24
In a study conducted by Lee et al. 25 the reconstruction of dural defects was performed using acellular human dermis as a substitute. The acellular human dermis offers distinct advantages compared to other dural substitutes. During the generation of acellular human dermis, all major histocompatibility class II antigens are eliminated, making it immunologically inert. This characteristic reduces the risk of immune reactions when used as a dural substitute. The extracellular structure of acellular human dermis promotes neovascularity, facilitating the formation of new dura mater tissue. This property is beneficial for the regeneration and repair of damaged dural tissue. Additionally, the acellular human dermis was found to be capable of repairing even large dural defects effectively. 25
Furthermore, Lifecell Inc, USA received FDA approval for a product called AlloDerm, which is an acellular human dermis graft used as a substitute for dura mater. Clinical studies have revealed the potential of AlloDerm to integrate with damaged dura mater tissue, facilitate cellular migration, and promote matrix deposition. AlloDerm has also been shown to prevent CSF leakage. These positive outcomes, along with its safety profile, have made AlloDerm a viable option for clinical applications in dural reconstruction and repair surgery.70,71 To summarize, the use of acellular human dermis as a dural substitute offers immunological inertness, promotes neovascularity, and enables the formation of new dura mater tissue. Clinical studies have demonstrated its effectiveness in repairing dural defects and preventing complications such as infection and CSF leakage. AlloDerm, an FDA-approved acellular human dermis graft, has shown promising results in terms of integration with damaged tissue and facilitating the healing process in dural reconstruction and repair surgeries.
Xenogeneic dural grafts
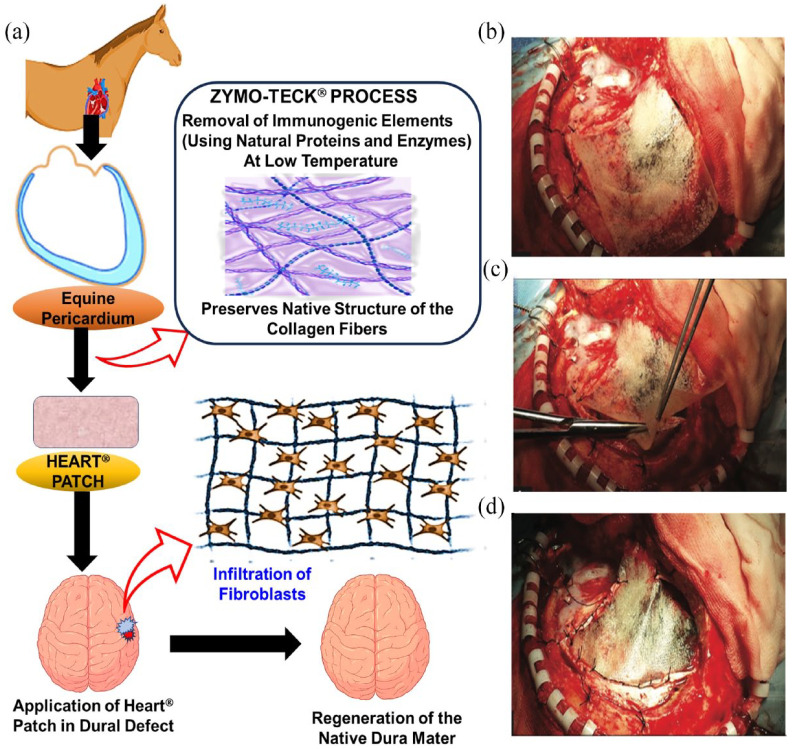
In addition to autologous and allogenic materials, xenogeneic dural substitutes derived from porcine, equine, bovine, or other animal tissues are used as alternatives for dural substitution. 68 A study conducted by Centonze et al. 26 focused on evaluating the efficacy and safety of the equine pericardium membrane, known as Heart membrane, for repairing dural defects resulting from meningioma surgery (Figure 6). 26 The equine pericardium membrane demonstrated its suitability for establishing a watertight closure of the dura mater at the site of the defect, effectively preventing CSF leakage. Notably, there were no instances of disease transmission observed, which is often a concern associated with other dural substitutes derived from animal tissue sources. This finding underscores the safety aspect of using the equine pericardium membrane as a xenogeneic substitute for the dura mater. Furthermore, the usage of pericardium from equine origin exhibited no adverse reactions or wound infections in the patients. This positive outcome suggests that the equine pericardium membrane holds significant potential as a robust and reliable xenogeneic substitute for the dura mater. Its ability to establish a secure closure, absence of disease transmission, and favorable safety profile make it a valuable option for dural substitution in clinical practice.
Figure 6.
Demonstration of the ZYMO-TECK® involved in the processing of Heart patch and the surgical process involved in the implantation: (a) illustrative diagram showcasing the equine pericardium membrane as a xenogeneic solution for replacing the dura mater. Heart® membrane employed as a dural alternative, (b) the membrane positioned over the defect, (c) the membrane cut into appropriate dimensions, and (d) the membrane sutured to the patient’s dura mater. 26 Copyright 2016, Thieme Medical Publishers.
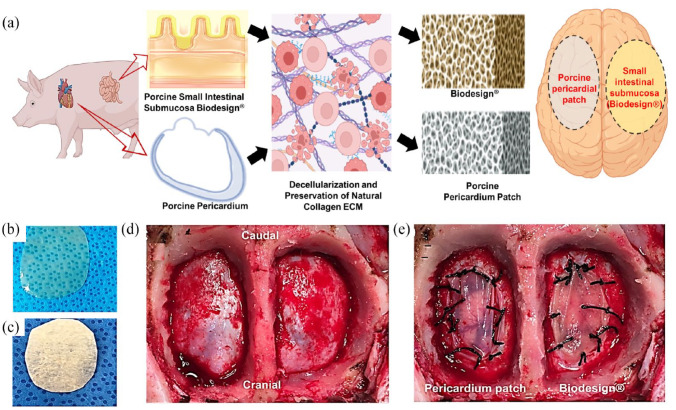
In a study conducted by Seo et al. 27 the effectiveness of porcine pericardium as a dural substitute for duraplasty was investigated using an animal model (pigs). The study aimed to compare the performance of porcine pericardium with that of porcine small intestinal submucosa (Biodesign®) as substitutes for the dura mater (Figure 7(a)). 27 The results of the study (Figure 7(d) and (e)) revealed that both porcine pericardium and porcine small intestinal submucosa provided a satisfactory watertight closure of the dura mater, indicating their potential as dural substitutes. Histologially, there was not a significant difference observed between the two substitutes. However, the dural substitute derived from porcine small intestinal submucosa exhibited better integration with the original dura mater. One advantage of using the porcine pericardium patch was its transparency, which can help prevent subdural hemorrhage following the duraplasty procedure. Also, porcine pericardium patches are more transparent as compared to commercially available Biodesign® (Figure 7(b) and (c)). This transparency allows for better visualization of the underlying tissues, reducing the risk of complications. It should be noted that this study had some limitations, such as a short observation period and a small sample size of only six pigs. Therefore, further studies with larger sample sizes and longer follow-up periods are necessary to fully evaluate the potential of porcine pericardium as a viable dural substitute. Overall, the study suggests that porcine pericardium holds promise as a dural substitute for duraplasty. However, additional research is needed to gather more evidence and validate its effectiveness and safety in diverse clinical settings.
Figure 7.
Illustrative depiction of the origin and morphology of pericardial patch and Biodesign® along with the duraplasty procedure: (a) schematic representation of pericardial patch and Biodesign® dural repair graft-based approach for duraplasty, (b) wet form of the porcine pericardial patch, (c) dry form of the Biodesign® Dural Repair Graft, (d) performance of bilateral craniotomies, and (e) duraplasty done using each type of dural substitute. 27 Copyright 2018, Society of Neuroscience.
Yu et al. 1 developed a novel substitute for the dura mater by utilizing the porcine peritoneal acellular matrix stabilized with oxidized quaternized guar gum (OQGG) as an antibacterial crosslinking agent. This substitute demonstrated antibacterial properties attributed to the presence of a quaternary ammonium group on the OQGG, which effectively damages the bacterial cell wall structure, leading to bacterial destruction. The constructed dura mater substitute exhibited good anti-adhesion properties and demonstrated compatibility with the surrounding tissue in in vivo studies. 1 Therefore, this matrix holds promise as a viable substitute for dural reconstruction.
Li et al. 10 fabricated a dura mater replacement by utilizing the acellular swim bladder of Hypophthalmichthys molitrix. 10 This substitute displayed a maximum tensile strength of 34.77 ± 4.28 N and stitch tear strength of 7.15 ± 1.84 N. In in vivo studies, the acellular swim bladder exhibited favorable biocompatibility, no cerebrospinal fluid leakage, and no adhesion with brain tissue. However, a slight inflammation response was observed, characterized by the growth of lymphocytes, migration of fibroblast cells, and deposition of connective tissue within the acellular swim bladder. This inflammation indicated non-specific inflammation rather than an adverse reaction. Overall, the acellular swim bladder demonstrated characteristics that make it a potential candidate for an ideal dura mater substitute. These studies highlight the development of innovative substitutes for the dura mater, such as the porcine peritoneal acellular matrix stabilized with OQGG and the acellular swim bladder of Hypophthalmichthys molitrix. These substitutes offer distinct properties, including antibacterial activity, anti-adhesion characteristics, histocompatibility, and strength. However, further research is necessary to validate their efficacy, safety, and long-term performance in clinical settings before their widespread use as dural substitutes.
Though traditional grafts have the advantage of being cost-effective and easily available, they have various limitations too. An autograft harvest could be inconvenient, especially if a donor site is not easily accessible and the need for a substitute wasn’t expected at the beginning of surgery. It should be highlighted that allografts and xenografts have the potential to spread infectious diseases, even if they are rare. Additionally, these grafts are from native tissues and can have wide variations in handling attributes and quality. Further research into polymeric dural substitutes with fewer immunogenic reactions and optimal dural replacement features is required due to these shortcomings.
Natural polymeric substitutes for the dura mater
Natural polymers have emerged as promising biomaterials for the development of grafts or tissue-engineered constructs, particularly for dural substitutes. Natural polymers possess greater interactions with the cells and have bioactive characteristics, which enables them to improve the growth and proliferation of the cells in a biological system. As a result, they do not cause foreign body reactions and are extremely biocompatible. Among these natural polymers, polysaccharide-based biopolymers such as chitosan and bacterial cellulose have garnered significant attention. Additionally, proteins like collagen and silk fibroin have also been investigated as potential biomaterials for dural substitutes (Table 2).
Table 2.
Summarized data of the natural polymeric dural substitutes with their advantages, limitations, study type, no. of subjects, mechanical properties, and anti-CSF leakage properties.
| Type of Dural substitute | Advantages | Limitations | Type of study | No. of subjects | Ultimate tensile strength (MPa) | Young’s modulus (MPa) | CSF leakage | References |
|---|---|---|---|---|---|---|---|---|
| Bacterial cellulose membrane (BCM) | Good porosity, inexpensive and reduces the possibility of secondary epilepsy and epidural scar formation | NA | In vitro and In vivo study (rabbits) | 42 | 9.70 ± 2.56 | 83.64 ± 22.04 | Not observed | Jing et al. 11 |
| GF induced bacterial cellulose membrane (BCM) | No inflammatory reaction seen and promotes the proliferation of cells | NA | In vitro and In vivo study (rats) | NA | 0.96 ± 0.02 | 0.37 ± 0.02 | NA | Stumpf et al. 28 |
| Vancomycin loaded bacterial cellulose membrane (BCM) | Inhibits inflammation, highly elastic and complies with pressure | NA | In vitro and In vivo study (rabbits) | 20 | NA | NA | NA | Xu et al. 29 |
| Bilayer Chitosan scaffold | Thin, inexpensive, promotes collagen fibers formation without any evidence of infection | NA | In vitro and In vivo study (rabbits) | NA | NA | NA | NA | Sandoval-Sánchez et al. 30 |
| Dura-Guard | Robust and can be used in sites with high CSF pressure | NA | In vivo study (hounds) | NA | 13.50 ± 3.34 | 81.33 ± 20.48 | Not observed | Zerris et al. 31 |
| Durepair | Robust and can be used in sites with high CSF pressure | NA | In vivo study (hounds) | NA | 22.70 ± 2.83 | 69.94 ± 9.49 | Not observed | Zerris et al. 31 |
| DuraGen | Effective in repairing dural defects | NA | In vivo study (hounds) | NA | NA | NA | Not observed | Zerris et al. 31 |
| TissuDura | Does not cause inflammatory reactions and does not adhere to surrounding tissue | NA | Clinical study | 208 | NA | NA | Not observed | Esposito et al. 32 |
| TissuDura | No adverse reactions observed | Relatively expensive | Clinical study | 47 | NA | NA | Observed in five patients | Pettorini et al. 33 |
| TissuDura | Demonstrates non-reactivity, elasticity, and good adaptability | NA | Clinical study | 74 | NA | NA | Not observed | Parlato et al. 72 |
| Silk fibroin based substitute | Inhibits inflammation | NA | In vitro and In vivo study (rats) | NA | NA | NA | Not observed | Kim et al. 34 |
| Silk fibroin based substitute | Inhibits CSF leakage and adhesion to surrounding tissue | NA | In vitro study | NA | 1.53 ± 0.45 | 12.1 ± 2.4 | NA | Flanagan et al. 35 |
Bacterial cellulose
Bacterial cellulose, a natural polysaccharide, has been extensively studied and derived from various bacteria such as Acetobacter, Rhizobium, and Sarcina. It possesses several advantageous features including easy extraction, high hydrophilicity, high purity, nanoscale characteristics, and favorable physical properties. In the field of medicine, there has been notable research focused on the utilization of bacterial cellulose.73,74 Jing et al. 11 conducted a study using bacterial cellulose membranes for reconstructing dural defects in rabbits. 11 The bacterial cellulose membranes were synthesized through electrospinning, resulting in membranes with enhanced porosity and water-holding capacity. These electrospun bacterial cellulose membranes exhibited a tensile strength of 9.70 ± 2.56 MPa and an elastic modulus of 83.64 ± 22.04 MPa. In in vivo studies, the electrospun bacterial cellulose membrane demonstrated the ability to prevent cerebrospinal fluid leakage and reduce the likelihood of secondary epilepsy and epidural scar formation. This was achieved by minimizing fibrous adhesion and inhibiting the attachment of muscle and collagen fibers to the outer surface of the graft, respectively. Thus, the electrospun bacterial cellulose membrane holds promise as an ideal substitute for the dura mater. 11 The utilization of natural polymers, including bacterial cellulose, in dural substitutes offers distinct advantages such as their abundance, biocompatibility, and tunable properties. These polymers provide an attractive alternative to traditional graft materials and hold potential for improving dural repair and reconstruction procedures. Further research and development are necessary to optimize the properties and performance of these natural polymer-based dural substitutes for their eventual clinical translation.
In addition to its use as a dural substitute, bacterial cellulose has been explored for various advanced applications. Stumpf et al. 28 developed a growth factor-loaded bacterial cellulose membrane for duraplasty. 28 This innovative dural substitute incorporated epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF2), which were released from the bacterial cellulose membrane to promote the proliferation of neural stem/progenitor cells. The growth factor-loaded bacterial cellulose membrane exhibited desirable mechanical properties, including an ultimate tensile strength of 0.96 ± 0.02 MPa and a Young’s modulus of 0.37 ± 0.02 MPa. In vivo studies further demonstrated that the use of the growth factor-loaded bacterial cellulose membrane as a dural substitute did not induce inflammation. 28 This suggests that the growth factor-loaded bacterial cellulose has the potential to serve as a promising substitute for the dura mater, particularly for conditions like spinal cord injuries and traumatic brain injuries, where growth factors can facilitate tissue regeneration and repair.
To address the challenge of post-operative infection, antibacterial drug-loaded bacterial cellulose membranes have also been extensively investigated. Xu et al. 29 developed a bacterial cellulose membrane containing vancomycin, an antibacterial drug, as a substitute for the dura mater. 29 This membrane was designed to release vancomycin during craniocerebral operations, aiming to minimize inflammatory reactions and prevent infection. In rabbit studies, the vancomycin-loaded bacterial cellulose membrane demonstrated safety and effectively inhibited inflammatory reactions in neurosurgical applications. 29 Moreover, it exhibited favorable elasticity and compliance with pressure. Thus, bacterial cellulose membranes not only serve as suitable substitutes for repairing dural defects but also hold promise as potential drug delivery vehicles, enabling localized and controlled release of therapeutic agents. The integration of growth factors and antibacterial drugs into bacterial cellulose membranes expands their functionality and enhances their therapeutic potential. These advancements offer opportunities for the development of more advanced and effective strategies for dural repair and reconstruction, as well as for the treatment of associated complications. Continued research in this field will contribute to the optimization and clinical translation of growth factor- and drug-loaded bacterial cellulose membranes for improved patient outcomes.
Chitosan
Chitosan, a glycosaminoglycan-like biopolymer derived from the deacetylation of chitin, which is derived from the shells of crustaceans and the exoskeleton of arthropods, has garnered significant attention from researchers for the development of tissue-engineered products due to its biomimetic properties. It possesses unique biological qualities such as biocompatibility, nontoxic biodegradability antimicrobial activity, minimal immunogenicity and toxicity that make it suitable for various applications, including the repair or reconstruction of defects in the central nervous system (CNS) caused by trauma or diseases.73 –81 One of the notable characteristics of chitosan is its ability to act as a chemoattractant for neutrophils, which are essential cells involved in the inflammatory response. By stimulating the recruitment of neutrophils and activating macrophages, chitosan can expedite the wound healing process. Additionally, chitosan derivatives have been found to regulate scar development and retraction during the healing process, thereby minimizing the formation of excessive scar tissue. These advantageous properties of chitosan have led to its extensive utilization in CNS-related applications. Researchers have explored its potential in the repair or reconstruction of CNS defects, such as those resulting from trauma or diseases.73 –81 Chitosan has been investigated for its ability to promote tissue regeneration, facilitate cell adhesion and migration, support angiogenesis (the formation of new blood vessels), and provide structural support in CNS tissues.
Sandoval-Sanchez et al. 30 conducted a study on the utilization of a bilayer chitosan scaffold as a novel substitute for the dura mater in neurosurgical procedures. 30 This scaffold consisted of two layers: a non-porous layer and a porous layer with a pore size of approximately 10 μm. The scaffold had a thickness of approximately 400 μm. One of the key findings of the study was that the bilayer chitosan scaffold provided an effective watertight closure of the dura mater. 30 Moreover, it demonstrated favorable properties such as suture compatibility and the ability to promote tissue regeneration without causing fibrosis. These characteristics make the bilayer chitosan scaffold an ideal candidate for dural substitutes.
Collagen
Collagen, another naturally derived material, is also extensively utilized in the development of dural substitutes due to its low antigenicity and high biocompatibility. It can undergo remodeling to enhance cellular behavior and tissue regeneration due to its dynamic nature and flexibility.82,83 Zerris et al. 31 evaluated three different collagen-based substitutes for dura mater reconstruction: Dura Guard, Durepair, and DuraGen. 31 Dura-Guard is a sturdy dural implant made from processed bovine pericardium sheets, which can be easily sutured to surrounding tissues. DuraGen is an onlay graft derived from bovine Achilles tendons, while Durepair is processed from fetal bovine skin. The study found that Dura Guard and Durepair exhibited superior strength compared to DuraGen, making them suitable for use in areas with high CS pressure. Dura Guard had a thickness of 0.40 ± 0.00 mm, a tensile strength of 13.50 ± 3.34 MPa, and a Young’s modulus of 81.33 ± 20.48 MPa. Similarly, Durepair had a thickness of 0.50 ± 0.02 mm, a tensile strength of 22.70 ± 2.83 MPa, and a Young’s modulus of 69.94 ± 9.49 MPa. All three collagen substitutes demonstrated safety and efficacy in repairing the dura mater. 31 The use of bilayer chitosan scaffolds provides a promising alternative for dural substitutes, offering effective closure and supporting tissue regeneration. Meanwhile, collagen-based substitutes like Dura Guard, Durepair, and DuraGen have proven to be safe and efficient options for dura mater reconstruction. These findings contribute to the development of reliable and biocompatible materials that can be employed in neurosurgical procedures to address dural defects effectively.
Esposito et al. 32 conducted a study to evaluate the safety and effectiveness of a collagen biomatrix derived from equine Achilles tendon, known as TissuDura, as a dural substitute. 32 TissuDura is primarily composed of type I collagen and has a lamellar structure without any pores, preventing the leakage of CSF and promoting the regeneration of the dura mater. According to histological investigations of the clinical study, the sample resembled a dura tissue that was richer in cellular components and tight fibres, essentially mimicking physiological dura mater. Fibroblasts cells were the first line of these cellular formations. Collagen fibres with a regular structure made up the extracellular matrix (ECM), with some minor abnormalities in their arrangement. It was possible to discern a clear neovascularization around the edges of the suspected transplant. Single mononuclear cell perivascular infiltrates were seen in these areas. The histopathological study also revealed no signs of necrotic tissue or foreign body reaction. The study demonstrated that the usage of TissuDura resulted in the absence of inflammatory reactions and adherence to surrounding tissues, making it a highly desirable dural substitute. 32 Pettorini et al. 33 further investigated the use of TissuDura in pediatric neurosurgical procedures. 33 The study highlighted several advantages of TissuDura, including ease of handling during implantation, the absence of inflammatory adverse reactions, and no observed CSF leakage. However, it was noted that TissuDura is relatively expensive compared to other dural substitutes. 33 In the same year, Parlato et al. 72 also assessed the performance of TissuDura as a dural graft in spinal and cranial neurosurgical procedures. 72 In this study, TissuDura was utilized as an onlay graft without the need for surgical sutures, using fibrin glue to secure it in place. Post-operation, no foreign body reactions, tissue adherence, or CSF leakage were observed. The qualities of TissuDura, including its elasticity, ease of usage, and non-reactivity, contribute to its suitability as an ideal dural substitute. 72 Collectively, these studies demonstrate the effectiveness of TissuDura as a dural substitute in various neurosurgical applications. Its unique composition and structural properties, along with its ability to prevent CSF leakage and promote tissue regeneration, make TissuDura a valuable option in neurosurgical procedures requiring dural reconstruction. However, the higher cost of TissuDura should be considered when selecting a dural substitute for clinical use.
Silk fibroin
Silk fibroin has emerged as a promising protein-based biomaterial for the development of tissue-engineered grafts as an alternative to collagen-based biomaterials. Researchers have shown great interest in utilizing silk fibroin (SF) for the construction of dural substitutes due to its excellent biocompatibility and unique mechanical properties. 78 Two proteins-hydrophilic sericin and hydrophobic fibroin-make up the SF biomolecule. Degradation and metabolism of SF result in the production of harmless amino acids. It has mechanical strength (which is greater than that of many other biological materials), elasticity, biocompatibility, and adjustable biodegradability. The characteristics of scaffolds made from SF can be altered by modifying its secondary structure. 84
Kim et al. 34 fabricated a novel dural substitute using silk fibroin derived from the Bombyx mori silkworm. 34 The manufactured dural substitute exhibited a tensile strength of 65.6 ± 7.1 MPa and underwent in vivo testing in a rat model. The study demonstrated that the silk fibroin-based substitute was safe for use in neurosurgical procedures, as it prevented inflammatory reactions and side effects. 34 Moreover, its transparency facilitated visualization during surgical procedures, reducing the risk of damage to underlying tissues. Flanagan et al. 35 developed a sutureless dural substitute using silk fibroin from Bombyx mori. 35 This substitute effectively inhibited CSF leakage and demonstrated excellent biocompatibility with no immune reactions. It consisted of electrogelated silk and a dual-layer silk composite material that mimicked the microenvironment of the human dura mater (Figure 8). The dual-layer composite acted as an impermeable barrier against CSF leakage, while the electrogelated layer served as an adhesive. The silk dural substitute exhibited high burst pressure resistance and had a tensile strength ranging from 0.88 ± 0.10 to 1.53 ± 0.45 MPa and a Young’s modulus ranging from 7.16 ± 0.66 to 12.1 ± 2.4 MPa. 35 To conclude, silk fibroin is a promising candidate for dural substitutes in terms of its biocompatibility, mechanical properties, and potential for surgical applications.
Figure 8.
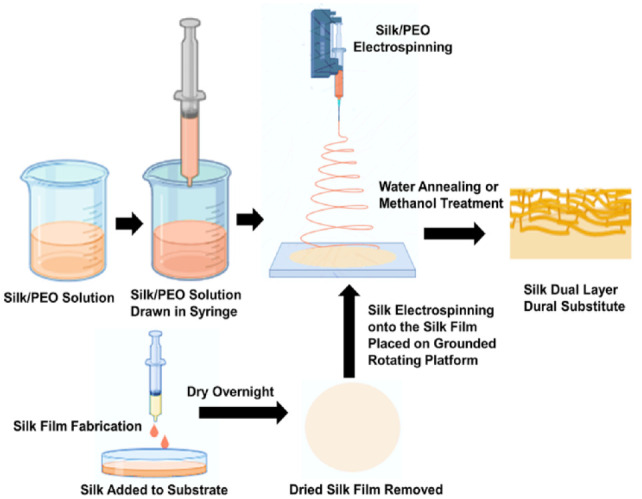
The production method for a dual-layer biomaterial composed of silk fibroin involves the following steps. 35
Although natural polymeric materials facilitate the formation of connective tissue at the site of dural injury and have good biocompatibility, they often face limitations, including batch-to-batch variability, rapid degradation, and weak mechanical strength.14,85 These restrictions make it challenging for the neo-dura mater to regenerate effectively.
Synthetic polymeric substitutes for dural mater reconstruction
Synthetic polymers have gained significant attention as potential substitutes for the dura mater in reconstructive procedures. These materials offer distinct advantages such as tunable properties, controlled degradation, and ease of fabrication.37,86 –89 The processing of these polymers into grafts allows for adequate control of the architectural parameters which include pore size and shape, wall morphology, and surface area, which are crucial for cell seeding, cell migration, cell growth, mass transport, and tissue regeneration. 83 Researchers have explored various synthetic polymers to develop effective substitutes for the dura mater, addressing the limitations associated with natural materials. To address the limitations such as poor mechanical properties, immune reactions, batch-to-batch variation, and scar formation associated with natural biopolymers, synthetic polymers have gained significant acceptance among researchers in the development of reproducible tissue-engineered grafts.
Researchers have explored various synthetic materials, including polycaprolactone (PCL), polyglycolic acid (PGA), polyurethane, and poly (L-lactic acid) (PLLA), for constructing dural biomimetic substitutes (Table 3). One of the advantages of synthetic polymers is their ease of fabrication, allowing for reproducible fabrication of dural substitutes. PCL and PLLA are examples of synthetic polymers with controllable degradation properties, making them suitable for dural reconstruction. These materials are also non-toxic and bioinert, reducing the risk of immune reactions and promoting biocompatibility. Polyurethane is another synthetic polymer investigated for dural replacements. While it offers excellent mechanical properties, it can induce foreign body reactions, limiting its applicability in some cases. However, ongoing research aims to overcome these challenges and optimize polyurethane-based dural substitutes. PGA, on the other hand, is often used as a PGA mesh combined with fibrin glue for repairing dural defects. PGA meshes exhibit high levels of tensile strength and can withstand the pressures within the central nervous system, effectively preventing cerebrospinal fluid (CSF) leakage. The unique properties of synthetic polymers make them promising candidates for dural substitutes. By utilizing synthetic polymers, researchers aim to develop reproducible tissue-engineered grafts with improved mechanical properties, reduced immune reactions, and minimized batch-to-batch variation. Further advancements in the field of synthetic polymers hold promise for the development of effective and reliable dural substitutes.37,86 –89
Table 3.
Summarized data of the synthetic polymeric dural substitutes with their advantages, limitations, study type, no. of subjects, mechanical properties, and anti-CSF leakage properties.
| Type of Dural substitute | Advantages | Limitations | Type of study | No. of subjects | Ultimate tensile strength (MPa) | Young’s modulus (MPa) | CSF leakage | References |
|---|---|---|---|---|---|---|---|---|
| Triple-layered PCL dural substitute | Mimics the extra cellular matrix of the native dura mater, prevents infections, and promotes tissue regeneration | NA | In vitro study | NA | 22.42 ± 0.89 | NA | NA | Su et al. 36 |
| Urethane linked PCL dural substitute | Effective integration with native dura mater and does not produce any inflammation or adhesion to surrounding tissues | NA | In vitro and in vivo study (rats) | NA | NA | NA | Not observed | Shih et al. 37 |
| PLLA patch | Good mechanical properties, no inflammation reaction and appropriate rate of degradation | NA | In vivo study (dogs) and clinical study | 24 dogs and 1 patient | 4.14 ± 0.18 | NA | Not observed | Shi et al. 16 |
| PGA mesh (GM111) | No inflammatory reaction and wound infection | NA | Clinical study | 60 | NA | NA | Not observed | Terasaka et al. 38 |
| PGA mesh | Effective in dural closure | Short follow-up time | Retrospective study | 75 | NA | NA | Observed in one patient | Masuda et al. 39 |
| PGA mesh | NA | Foreign body granuloma was observed | Case report | 1 | NA | NA | NA | Kawabata et al. 40 |
| Dural sealant patch | Effective in dural closure and preventing CSF leakage | One case of serious adverse event was reported | Clinical study | 40 | NA | NA | Not observed | Van Doormaal et al. 41 |
| Neuro-patch | No incidence of infection seen | NA | Clinical study | 103 | NA | NA | Not observed | Li et al. 42 |
| Neuro-patch | NA | Raised risk of wound infection | Retrospective study | 61 | NA | NA | 13% | Malliti et al. 43 |
Polycaprolactone (PCL)
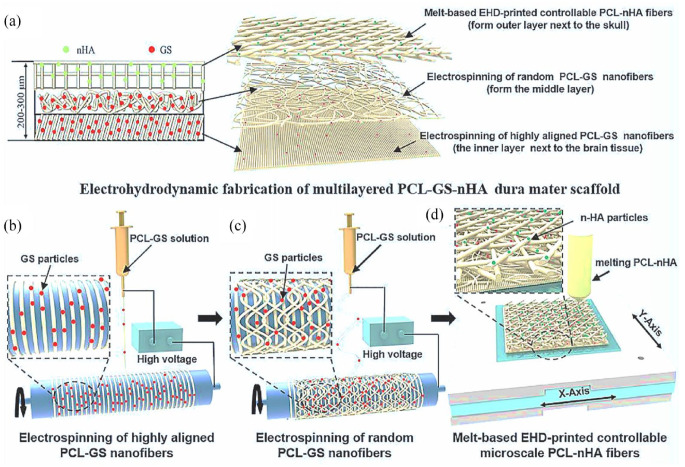
The synthetic polymer PCL has emerged as a promising material for fabricating alternative matrices for the dura mater, the protective membrane surrounding the brain and spinal cord. PCL possesses several advantageous properties, including good biocompatibility, biodegradability, low cost, and the ability to prevent CSF leakage by integrating with the surrounding tissues.90,91 In a study by Su et al., 36 a triple-layered dural substitute using PCL was developed with enhanced antibacterial properties and osteogenic capabilities. 36 The scaffold incorporated gentamicin sulfate (GS) and nano-hydroxyapatite (nHA), which played vital roles in inhibiting bacterial growth and promoting the formation of bone-like tissue. The fabrication technique employed electrohydrodynamic jetting, with PCL-GS fibers electrospun at different angles to form the middle layers of the substitute, enhancing its mechanical properties. Additionally, PCL-nHA fibers were constructed using the melt-based electrohydrodynamic technique to form the cranial side of the dural substitute (Figure 9). 36 The resulting dura mater substitute exhibited a tensile strength of 22.42 ± 0.89 MPa, which closely resembles that of the natural porcine dura mater. However, further in vivo studies are required to validate the effectiveness of this PCL-based substitute. 36
Figure 9.
Schematic diagram of the fabrication of triple-layered PCL dura mater substitute (loaded with GS and nHA): (a) electrohydrodynamic fabrication of PCL-GS-nHA dura mater scaffold, (b) electrospinning of highly aligned PCL-GS nanofibers, (c) electrospinning of random PCL-GS nanofibers, and (d) fabrication of melt-based microscale PCL-nHA fibers. 36 Copyright 2022, Elsevier.
Another noteworthy study by Shih et al., 37 reported the development of a urethane-linked PCL dural substitute. 37 This substitute demonstrated improved mechanical properties and a more favorable hydrolytic degradation process compared to pure PCL. The histological findings show a brief initial tissue reaction that is temporary and is followed by the fibrous, connective tissue layers deposition. The dural substitute’s porous structure permits the implant to be gradually integrated by the newly developed fibrous, connective tissue after 3 months of implantation in rabbits, with a low risk of inflammation and CSF leakage. Additionally, there was no evidence of dural substitute’s tissue attachment to the surrounding tissue. Overall, it exhibited biocompatibility, flexibility, and was effective in preventing inflammation and scar formation. Although further clinical research is necessary, this PCL-based substitute shows promise as a potential alternative matrix for the dura mater. 37 Overall, PCL-based dural substitutes offer advantages such as biocompatibility, degradability, and the ability to inhibit CSF leakage. Their ability to be fabricated with enhanced properties, such as antibacterial activity and osteogenic capabilities, makes them attractive options for dura mater replacements. Further research and in vivo evaluations will provide a better understanding of their efficacy and safety in clinical applications.
Poly-L-lactic acid (PLLA)
Poly-L-lactic acid (PLLA) is widely utilized in biomedical applications due to its excellent biodegradability and biocompatibility. It has been shown to effectively repair dural lesions by preventing CSF leakage and integrating with the surrounding tissue.90,92 In a study by Shi et al., 2016, a PLLA-based dural substitute was constructed by depositing PLLA fibers in a layer-by-layer fashion to mimic the extracellular matrix of the natural human dura mater. 16 The resulting PLLA-based patch exhibited a tensile strength of 4.14 ± 0.18 MPa. This biomimetic patch demonstrated remarkable biocompatibility, prevented virus transmission, and provided effective watertight dural closure, promoting appropriate healing. The biomimetic patch blended in effectively with the surrounding tissues, without leaving noticeable traces of the boundaries. There were no overall adhesions with brain tissue except in a few places where mild tissue adhesion was seen. Ninety days after implantation, the biomimetic patch was entirely wrapped in the surrounding tissues, substituted with connective dura tissue that had been heavily fibroblast-infiltrated and showed substantial neovascularization. By the completion of 180 days, the PLLA-based patch was 70% degraded with the degraded part covered with collagen fibers and new vessels. Furthermore, this patch was determined to be safe and effective, completely degrading after two years of implantation.
Polyglycolic acid (PGA)
Another notable synthetic polymer used for dural repair is polyglycolic acid (PGA). 93 PGA mesh combined with fibrin glue has been evaluated for repairing dural defects in animal models such as beagles and rabbits. This approach offers a relatively simple procedure, making it a suitable alternative for the dura mater. 93 Terasaka et al. 38 investigated the effectiveness and safety of a sutureless dural substitute composed of polyglycolic acid mesh and fibrin glue (GM111). 38 The substitute exhibited good biocompatibility, enhanced safety, and provided a water-tight dural closure, effectively protecting patients from surgical complications. Similarly, Masuda et al. 39 repaired dural defects in spinal surgery using a combination of polyglycolic acid mesh and fibrin glue. 39 This combination demonstrated the ability to withstand high levels of CSF pressure, effectively preventing CSF leakage. After the procedure, the PGA mesh in the combination is absorbed in 8 weeks and the fibrin glue gets substituted by connective tissue in 4 weeks. In a clinical study involving 75 patients, only 1 patient experienced CSF leakage, indicating the potential of polyglycolic acid mesh and fibrin glue as an artificial dura mater. 39
However, it’s important to note that complications can arise in some cases. Kawabata et al. 40 reported a case of granuloma formation following the use of a combination of polyglycolic acid mesh and fibrin glue for dural defect repair. 40 Histopathological analysis of the removed granuloma showed that eosinophils had infiltrated the polyglycolic acid mesh fibre, which was encircled by multi-nucleated giant cells and histiocytes. These results indicated that a foreign body reaction had occurred due to the polyglycolic acid mesh. This granuloma caused compression of the cervical cord, leading to various complications such as extreme muscle weakness and numbness. Therefore, it is imperative to discover a safer substitute that results in a reduced immune response. In summary, PLLA and PGA have shown promise as synthetic polymeric materials for dural substitutes. PLLA-based patches exhibit excellent biocompatibility and degrade over time, while PGA mesh combined with fibrin glue offers a simple and effective solution for dural defect repair. However, careful evaluation and monitoring are necessary to minimize the risk of complications and ensure successful clinical outcomes.
Polyurethane
Polyurethane has emerged as a promising material for the fabrication of grafts used in the repair of the dura mater. This synthetic material is composed of a highly purified polyesterethane substance with fine fibers, providing flexibility to the implant. Polyurethane is commonly employed in brain and spinal surgery to address dural abnormalities due to its high liquid tightness, effectively inhibiting CSF leaks. 94 A study by Doormaal et al. 41 investigated the performance and efficacy of a dural sealant patch (DSP) in cranial surgeries. 41 The DSP consisted of two layers: a white adhesive layer composed of a bioresorbable copolyester and a blue sealing layer made of polyurethane. The researchers observed that none of the patients experienced postoperative CSF leakage. However, six patients reported serious adverse events such as pulmonary embolism, pneumonia, dyspnea, dysphasia, heartstroke which further led to renal implantation, urosepsis, incidences of high sodium, epilepsy, subdural hematoma, hypothalaam syndrome, chemical meningitis, viral eye infection, and appendicitis. However, only one of these occurrences-chemical meningitis-possibly had a direct connection to the DSP which the person developed following a craniotomy. Additionally, two patients developed pseudomeningocele, but these cases did not have any clinical repercussions as the pseudomeningocele was self-limiting and were resorbed eventually. Overall, the findings suggest that the DSP is a potentially effective device for controlling CSF leakage during intracranial surgery, making it a viable substitute for the dura mater. 41
In another study by Li et al., 42 the safety and efficacy of a polyurethane-based dural substitute called Neuro-Patch were investigated in microvascular decompression (MVD) surgery. Neuro-Patch is a popular dural substitute made from polyurethane. The results showed that Neuro-Patch effectively prevented CSF leakage, and no incidences of infection were observed during wound healing. 42 Neuro-Patch exhibits promising anti-CSF leakage and dura mater regeneration properties in terms of both safety and efficacy. However, it is worth noting that Malliti et al. 43 concluded that the use of Neuro-Patch as an artificial dura mater could lead to an increased risk of wound infections such as meningitis, empyema, and osteitis. 43 It can be concluded from this investigation that Neuro-Patch poses a higher risk for deep wound infections which can further have serious repercussions on a patient’s health. 43 This highlights the importance of careful evaluation and consideration of potential risks associated with the use of polyurethane-based dural substitutes. In summary, polyurethane-based materials, such as the dural sealant patch (DSP) and Neuro-Patch, show promise in controlling CSF leakage and providing effective substitutes for the dura mater. However, further research and evaluation are necessary to ensure their safety and minimize the risk of complications, such as wound infections.
Overall, synthetic materials provide the advantages of being readily available, being able to be shaped, and being able to be made with consistent handling qualities due to their manufacturing process. Moreover, there is no chance of disease transmission because they do not originate from biological sources. However, they have the disadvantage of being inefficient cell carriers, which is essential for tissue regeneration. The drawbacks of using natural and synthetic polymers separately can be overcome by developing composite alternatives that combine both types of polymers which can potentially make an ideal dural replacement.
Composite polymeric dural grafts: Enhancing dural repair with combined materials
Enhancing dural repair with composite polymeric grafts: combining the best of natural and synthetic materials composite matrices composed of both natural and synthetic polymer materials have emerged as promising options for the development of dural substitutes. By harnessing the unique properties of each material, these composites can be tailored to meet the desired physico-chemical, mechanical, and biological requirements for dura mater reconstruction and repair (Table 4). Natural materials offer several advantages in dural substitute design. They serve as excellent carriers for cells, promoting dural regeneration and facilitating cell adhesion. Additionally, natural polymers can help regulate the host immune response, minimizing adverse reactions. On the other hand, synthetic materials provide the ability to mimic the mechanical properties of native dural tissue, ensuring optimal performance.95,96
Table 4.
Summarized data of the composite polymeric dural substitutes with their advantages, limitations, study type, no. of subjects, mechanical properties, and anti-CSF leakage properties.
| Type of Dural substitute | Advantages | Limitations | Type of study | No. of subjects | Ultimate tensile strength (MPa) | Young’s modulus (MPa) |
CSF leakage | References |
|---|---|---|---|---|---|---|---|---|
| PLGA/type I collagen/chitosan | Reduced inflammatory reactions and faster restoration of neurological functions | Higher incidence of CSF leakage | In vivo study (rabbit) | 6 | NA | NA | 22.2% | Bai et al. 18 |
| PLLA/Gelatin | No incidence of infection seen and appropriate wound healing observed | NA | In vitro and in vivo study (dogs) and clinical study | 15 dogs and 5 patients | 3.8 ± 0.34 | NA | Not observed | Deng et al. 20 |
| ORC/PCL (P10) | No evidence of implant rejection and adhesion to surrounding tissues | Slight chances of CSF leakage and foreign body reaction | In vitro and in vivo study (rabbits) | 15 | 5.85 ± 0.27 | NA | Not observed | Chumnanvej et al., 44 Hemstapat et al., 45 and Sanpakitwattana et al. 46 |
| ORC/PCL (P20) | No evidence of implant rejection and adhesion to surrounding tissues | Slight incidence of foreign body reaction | In vitro and in vivo study (rabbits) | 15 | 2.79 ± 0.14 | NA | Not observed | Chumnanvej et al., 44 Hemstapat et al., 45 and Sanpakitwattana et al. 46 |
| PLLA/Gelatin/Chitosan | Demonstrated antibacterial activity and inhibited adhesion to surrounding tissues | NA | In vitro and In vivo study (rats) | 12 | 0.366 ± 0.002 | NA | Not observed | Liao et al. 47 |
One notable study by Bai et al. 18 explored a composite substitute for dura mater reconstruction, combining poly(lactic-co-glycolic acid) (PLGA), type I collagen, and chitosan. 18 The porous nature of PLGA facilitated tissue infiltration and cellular migration, while collagen and chitosan contributed to enhanced biocompatibility, cell adhesion, and proliferation. The composite substitute was evaluated in rabbit models with dural defects, demonstrating faster restoration of neurological functions and reduced inflammatory reactions. These findings highlight the biocompatibility and potential of this composite as an effective dural substitute. 18 Another composite dural substitute developed by Deng et al. 20 involved a combination of poly(L-lactic acid) (PLLA) and gelatin. 20 This composite exhibited improved mechanical properties, with notable tensile strength 3.8 ± 0.34 MPa and suture retention strength 3.03 ± 0.12 N. In vivo studies conducted in adult dogs confirmed the integration of the composite substitute with the native dura mater. The fibrous-porous structure of the substitute facilitated cell adhesion and migration, promoting faster regeneration of neo-dural tissue. Moreover, the hydrophobic and negatively charged surface of the composite, attributed to PLLA, effectively prevented CSF leakage. Clinical studies involving five patients further validated the composite substitute’s efficacy, as no instances of CSF leakage or infections were reported. 20 By combining the strengths of natural and synthetic polymers, composite polymeric grafts offer a promising approach to enhance dural repair. These grafts can provide optimal biocompatibility, mechanical properties, cell adhesion, and tissue regeneration. Continued research and clinical studies are necessary to further evaluate the performance and safety of these composite substitutes, bringing us closer to effective alternatives for the dura mater.
The mechanical properties of the dura mater, characterized by interwoven collagen fibers, contribute to its robustness. To mimic these properties, Hempstapat et al. 45 developed a bilayer knitted fabric-reinforced composite dural substitute using oxidized regenerated cellulose (ORC) and PCL. 45 ORC is generated by oxidizing cellulose with nitrogen dioxide and subsequent regeneration. The composite was prepared in two formulations: P10 and P20, containing 10 and 20 g of PCL, respectively. The tensile strength of P10 was measured at 5.85 ± 0.27 MPa, while P20 exhibited a lower tensile strength of 2.79 ± 0.14 MPa. The composite structure consisted of microporous and dense regions, closely resembling the human dura mater. P20 demonstrated complete inhibition of CSF leakage, whereas slight leakage was observed with P10. 45 To evaluate the biocompatibility and performance of the composite substitute, Chumnanvej et al. 44 conducted in vivo studies in rabbits. 44 A total of 45 rabbit models were used, and both P10 and P20 formulations exhibited biocompatibility without inducing adverse reactions, although a mild foreign body reaction was observed. Regeneration of new dura mater was observed after 1 month of implantation as indicated by the formation of dense network of collagen fibers, with P10 demonstrating a faster degradation rate compared to P20. A histological analysis of the interface between the material and the brain tissue revealed that there was very little adhesion between ORC/PCL composites and the brain tissue. These findings suggest that the bilayer ORC/PCL composite dural substitute holds promise as an alternative artificial dura mater.
However, further clinical studies are required to gain deeper insights into its performance. 44 In subsequent research, Sanpakitwattana et al. 46 made modifications to the same bilayer ORC/PCL composite substitute by incorporating cefazolin in varying amounts to impart antibacterial properties. 46 Cefazolin is known for its efficacy against bacteria such as Staphylococcus aureus and coagulase-negative staphylococci, commonly associated with post-operative surgical site infections and post-craniotomy infections. Among different formulations with varying cefazolin concentrations, the P20 formulation containing 2.5 g of cefazolin exhibited the most promising antibacterial properties, lasting up to 4 days in vitro. Importantly, the mechanical and physical properties of the composite remained comparable to the original ORC/PCL composite without the drug. Further, in vivo studies are necessary to validate the scaffold’s ability to release the drug at the site of dural injury and its efficacy in preventing infections. 46 By combining collagen-mimicking properties, biocompatibility, mechanical strength, and antibacterial capabilities, the bilayer ORC/PCL composite dural substitutes offer a potential solution for dural repair.
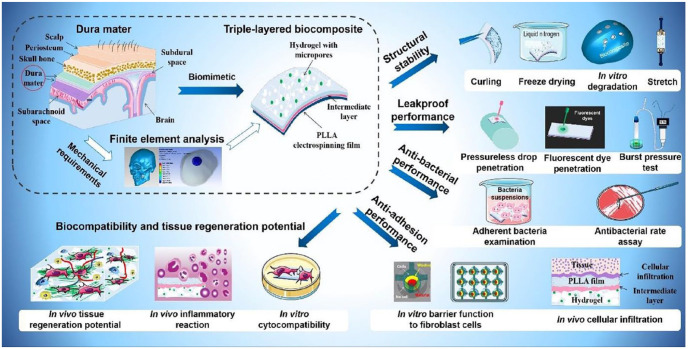
These advancements pave the way for improved artificial dura mater alternatives, although further research and clinical investigations are needed to validate their effectiveness and safety in clinical settings. Figure 10 represents the schematic diagram of the various features displayed by the biomimetic tri-layered dural substitute, including structural stability, antibacterial, antiadhesive traits, and tissue regeneration potential. 47 In the pursuit of an effective dural substitute developed a three-layered composite composed of PLLA, gelatin, chitosan, and acellular small intestinal submucosa (SIS) powder. 47 This composite exhibited several desirable characteristics, including the potential for dura regeneration, antimicrobial properties, and prevention of leakage and meningocerebral adhesion (Figure 10). The tensile strength of the composite was measured at 366 ± 2 kPa, indicating its mechanical resilience. The incorporation of SIS powder in the substitute played a crucial role in promoting cell proliferation, while gelatin contributed to the formation of a porous structure, facilitating cell growth within the substitute. PLLA contributed to the hydrophobic surface of the composite, effectively preventing CSF leakage. Additionally, chitosan, known for its antibacterial properties, endowed the substitute with the ability to combat microbial growth. The multifunctional nature of this composite holds promises for dura mater repair. 47 It not only addresses the mechanical requirements but also promotes dura regeneration, prevents CSF leakage, and possesses antimicrobial properties. By combining the beneficial characteristics of each component, this three-layered dural substitute composite offers a potential solution for enhancing the effectiveness of dura mater repair procedures.
Figure 10.
Systematic images show that the triple-layered composite has effective multifunctionality, including leak-proof ability, antiadhesion performance, antibacterial ability, and dura regeneration potential. 47 Copyright 2021, Elsevier.
The studies on composite grafts highlight that they offer ideal dural substitute characteristics but the clinical investigation of the composite dural substitutes is in its initial phase. Thus, further research and clinical studies are needed to evaluate its performance and safety in vivo and validate its potential application in clinical settings.
In order to assess the mechanical properties of various dural substitutes, the ultimate tensile strength and Young’s modulus have been examined and compiled in Tables 1 to 4. The findings are also visualized in Figure 11.
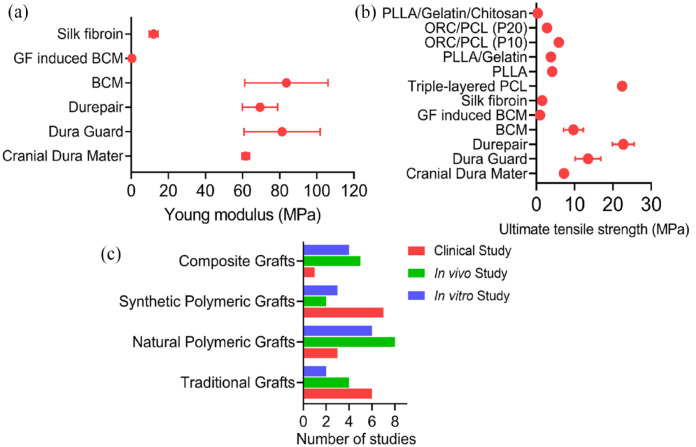
Figure 11.
Graphical representation of the mechanical properties and proportion of studies performed on various types of grafts: (a) ultimate tensile strength for various dural substitutes,11,16,20,28,31,35,36,45,47,97 (b) Young’s modulus for different dural substitutes,11,28,31,35,97 and (c) ratio clinical, in vivo and in vitro study for the dural grafts.1,10,11,16,18,21 –47,72
Figure 11a and b, illustrating the variations in ultimate tensile strength and Young’s modulus, respectively. Based on the study, it can be inferred that while Durepair exhibits the highest ultimate tensile strength, both Bacterial Cellulose membrane and ORC/PCL (P10) closely approximate the ultimate tensile strength of the natural cranial dura mater. These two substitutes, therefore, hold promise as potential ideal alternatives in terms of ultimate tensile strength. Regarding Young’s modulus, only Durepair, DuraGuard, and Bacterial Cellulose membrane demonstrate values similar to the native cranial dura mater. This indicates that these materials possess comparable stiffness and elasticity characteristics. Furthermore, Figure 11(c) provides an overview of the proportion of clinical, in vivo, and in vitro studies conducted on conventional, natural polymeric, synthetic polymeric, and composite dural substitutes. The graph reveals that a greater number of studies have been conducted on traditional and polymeric dural substitutes in each domain, whereas research on composite dural substitutes is relatively scarce. This suggests that investigations into composite grafts are still in their early stages. As a result, further research on composite grafts is necessary to ascertain their effectiveness in dura mater repair. In summary, the evaluation of tensile strength and Young’s modulus of dural substitutes provides valuable insights into their mechanical properties. Identifying substitutes that closely resemble the mechanical characteristics of the native dura mater is crucial for achieving optimal performance and outcomes in dura mater repair. Continued research and exploration of composite grafts will contribute to expanding our understanding of their potential and refining their application in dura mater reconstruction.
Dural sealants for the prevention of CSF leakage
Cerebrospinal fluid (CSF) leakage is a frequently occurring postoperative consequence of neurosurgical treatments. Pneumocephalus, meningitis, epidural infections, and slower healing of wounds are repercussions of CSF leakage. These complications can have serious consequences on the patient’s health which can lead to reoperation in some cases. The size of the dural opening, as well as the location and purpose of the surgery, can affect the frequency of CSF leakage. In general, reports of CSF leaks during cranial surgery range from 4% to 32%. To prevent CSF leakage during neurosurgery procedures, efficient and effective dural closure is essential.98 –101 Commercially accessible dural sealants seek to lower the risk of CSF leakage by strengthening the dural closure. The majority of these sealants were initially created as hemostatic agents. These hemostatic agents have characteristics of barrier function, tissue adhesion and absorption over time which makes them an ideal candidate for dural sealing. 102
Typically, there are two kinds of dural sealants: biological absorbable sealants that contain autogenic or allogenic fibrogen combined with thrombin, and synthetic absorbable sealants that contain PEG-based polymers. Both varieties are offered as patches and in liquid form. Dural sealants can be used when CSF leakage occurs following primary closure. Alternatively, they can be used in conjunction with a wide range of dural replacements to aid in closing the dural defect. 103 Ease of fabrication, sterilization, and handling are three characteristics that make a dural sealant an ideal one. It should be able to quickly create a proper watertight seal and preserve it. In addition, it should be flexible but robust, nontoxic, chemically inert, and should not trigger inflammatory responses. A cost-effective solution that does not cause infections or adhesions is the ideal dural sealant.104,105,106
Conclusion and future perspectives
The development of dural substitutes for repairing the dura mater has made significant strides, but there is still a need for the creation of an ideal dural substitute that can mimic the desired physico-chemical, mechanical, and biological properties. Composite materials have shown promise in this regard, offering the potential for tunable characteristics that closely resemble the native dura mater. However, the complex fabrication processes involved in creating these materials have limited their widespread usage, and further experimental investigations are necessary. One of the key challenges is the development of a multi-layered leakage-proof regenerative matrix. Ideally, a dural substitute should possess properties that prevent CSF leakage, promote tissue regeneration, and closely resemble the structure of the native dura mater. Composite materials have the potential to meet these criteria by combining the advantages of different components, such as enhanced mechanical strength, cell adhesion, and antibacterial properties. Continued advancements in composite materials offer a promising avenue for the creation of an ideal dural substitute in the future.
Furthermore, there is a need for more comprehensive studies on dura mater regeneration using various substitutes and a better understanding of the host body’s response to these materials. Research efforts should focus on investigating the interaction between cells, materials, and bioactive compounds to optimize the design and composition of dural substitutes. This knowledge will contribute to improving existing substitutes and guiding the development of future solutions for dural repair. Another important aspect is the individualized nature of dural repair. Current dural substitutes follow a one-size-fits-all approach, lacking customization to meet the specific needs of each patient. A 3D printing technology holds promise in addressing this limitation by enabling the fabrication of substitutes that closely resemble the intricate structures of native dura tissue. With the incorporation of cells, growth factors, and other bioactive compounds, 3D-printed dural substitutes have the potential to enhance healing and provide tailored solutions for individual patients. For instance, it is possible to replicate the multilayered structure of dura mater by creating distinct layers using various 3D printing models in accordance with each layer’s native structure. Furthermore, different cells can be incorporated in separate layers via 3D printing. For example, osteoblasts can be added to the layer of the substitute that faces the periosteum, while fibroblasts can be added to the meningeal layer. The integration of 3D printing techniques with composite materials and regenerative strategies represents a significant advancement in the future development of optimal dural repair substitutes. A variety of combinations of natural and synthetic polymers can be employed in the layer-by-layer 3D printing method to create customisable scaffolds with intricate shapes that can replicate the extracellular matrix and have uniform cell distribution. Additionally, various growth factors and antibiotics can be encapsulated in the substitutes fabricated through 3D printing to facilitate the effective regeneration of the dura mater as well as the prevention of wound infections.
In summary, while significant progress has been made in the field of dural substitutes, there is still a need for the development of an ideal substitute that possesses leakage-proof properties and mimics the native dura mater’s characteristics. The utilization of composite materials, along with advancements in 3D printing and regenerative strategies, shows promise in addressing these challenges. Further research, experimentation, and personalized approaches are required to advance the field and provide improved dural repair solutions for patients in the future.
Footnotes
Authors contributions: Dolphee Khurana and Ankitha Suresh contributed equally to the drafting of the manuscript. All authors contributed to the writing process and provided their comments and feedback on the preparation and improvising the manuscript. Finally, all authors reviewed and approved the final version of the manuscript before its submission or publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Manipal School of Life Sciences, Manipal Academy of Higher Education. The authors are also thankful to the SERB, Government of India for providing support through SRG funding (SRG/2021/001686) and Manipal Academy of Higher Education for providing support through Intramural funding (MAHE/CDS/PHD/IMF/2023). This work was supported by National Research Foundation of Korea (NRF) grants, Republic of Korea (2021R1A5A2022318, 2018K1A4A3A01064257, and RS-2023-00247485).
ORCID iDs: Jonathan C Knowles  https://orcid.org/0000-0003-3917-3446
https://orcid.org/0000-0003-3917-3446
Hae-Won Kim  https://orcid.org/0000-0001-6400-6100
https://orcid.org/0000-0001-6400-6100
Rajendra K Singh  https://orcid.org/0000-0003-3127-3346
https://orcid.org/0000-0003-3127-3346
References
- 1. Yu X, Yue P, Peng X, et al. A dural substitute based on oxidized quaternized guar gum/porcine peritoneal acellular matrix with improved stability, antibacterial and anti-adhesive properties. Chin Chem Lett 2023; 34: 107591. [Google Scholar]
- 2. Chuan D, Wang Y, Fan R, et al. Fabrication and properties of a biomimetic dura matter substitute based on stereocomplex poly (Lactic acid) nanofibers. Int J Nanomedicine 2020; 15: 3729–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song Y, Li S, Song B, et al. The pathological changes in the spinal cord after dural tear with and without autologous fascia repair. Eu Spine J 2014; 23: 1531–1540. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Ao Q. Research and application progress on dural substitutes. J Neurorestoratol 2019; 7: 161–170. [Google Scholar]
- 5. Jackson N, Muthuswamy J. Artificial dural sealant that allows multiple penetrations of implantable brain probes. J Neurosci Methods 2008; 171: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Velnar T, Gradisnik L. Soft tissue grafts for dural reconstruction after meningioma surgery. Bosn J Basic Med Sci 2019; 19: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abuzayed B, Kafadar AM, Oğuzoğlu ŞA, et al. Duraplasty using autologous fascia lata reenforced by on-site pedicled muscle flap: technical note. J Craniofac Surg 2009; 20: 435–438. [DOI] [PubMed] [Google Scholar]
- 8. Tachibana E, Saito K, Fukuta K, et al. Evaluation of the healing process after dural reconstruction achieved using a free fascial graft. J Neurosurg 2002; 96: 280–286. [DOI] [PubMed] [Google Scholar]
- 9. Li Q, Mu L, Zhang F, et al. A novel fish collagen scaffold as dural substitute. Mater Sci Eng C Mater Biol Appl 2017; 80: 346–351. [DOI] [PubMed] [Google Scholar]
- 10. Li Q, Zhang F, Wang H, et al. Preparation and characterization of a novel acellular swim bladder as dura mater substitute. Neurol Res 2019; 41: 242–249. [DOI] [PubMed] [Google Scholar]
- 11. Jing Y, Ma X, Xu C, et al. Repair of dural defects with electrospun bacterial cellulose membranes in a rabbit experimental model. Mater Sci Eng C Mater Biol Appl 2020; 117: 111246. [DOI] [PubMed] [Google Scholar]
- 12. Santiago G, Wolff A, Huang J, et al. Dural reconstruction with autologous rectus fascia. J Craniofac Surg 2019; 30: 326–329. [DOI] [PubMed] [Google Scholar]
- 13. Kizmazoglu C, Aydin HE, Kaya I, et al. Comparison of biomechanical properties of dura mater substitutes and cranial human dura mater: an in vitro study. J Korean Neurosurg Soc 2019; 62: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Wang W, Liao J, et al. Novel bilayer wound dressing composed of SIS membrane with SIS cryogel enhanced wound healing process. Mater Sci Eng C Mater Biol Appl 2018; 85: 162–169. [DOI] [PubMed] [Google Scholar]
- 15. Shivapathasundram G, Stoodley MA. Use of a synthetic dural substitute to prevent ventral retethering in the management of diastematomyelia. J Clin Neurosci 2012; 19: 578–581. [DOI] [PubMed] [Google Scholar]
- 16. Shi Z, Xu T, Yuan Y, et al. A new absorbable synthetic substitute with biomimetic design for dural tissue repair. Artif Organs 2016; 40: 403–413. [DOI] [PubMed] [Google Scholar]
- 17. Lecina-Tejero Ó, Pérez MÁ, García-Gareta E, et al. The rise of mechanical metamaterials: Auxetic constructs for skin wound healing. J Tissue Eng 2023; 14: 20417314231177838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. sBai W, Wang X, Yuan W, et al. Application of PLGA/type I collagen/chitosan artificial composite dura mater in the treatment of dural injury. J Mater Sci Mater Med 2013; 24: 2247–2254. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Guo H, Ying D. Multilayer scaffold of electrospun PLA-PCL-collagen nanofibers as a dural substitute. J Biomed Mater Res B Appl Biomater 2013; 101: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 20. Deng K, Yang Y, Ke Y, et al. A novel biomimetic composite substitute of PLLA/gelatin nanofiber membrane for dura repairing. Neurol Res 2017; 39: 819–829. [DOI] [PubMed] [Google Scholar]
- 21. Zeng T, Wang M, Xu Z, et al. Autologous free fascia lata can be used as dura graft in the salvage treatment of recalcitrant postcraniotomy intracranial infection caused by multidrug-resistant gram-negative bacteria. Infect Drug Resist 2022; 15: 5667–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffman H, Bunch KM, Paul T, et al. Comparison of pericranial autograft and alloderm for duraplasty in patients with type i chiari malformation: retrospective cohort analysis. Operat Neurosurg 2021; 21: 386–392. [DOI] [PubMed] [Google Scholar]
- 23. Marton E, Giordan E, Gallinaro P, et al. Homologous amniotic membrane as a dural substitute in decompressive craniectomies. J Clin Neurosci 2021; 89: 412–421. [DOI] [PubMed] [Google Scholar]
- 24. Eichberg DG, Richardson AM, Brusko GD, et al. The use of dehydrated amniotic membrane allograft for augmentation of dural repair in transsphenoidal endoscopic endonasal resection of pituitary adenomas. Acta Neurochir 2019; 161: 2117–2122. [DOI] [PubMed] [Google Scholar]
- 25. Lee JH, Choi SK, Kang SY. Reconstruction of chronic complicated scalp and dural defects using acellular human dermis and latissimus dorsi myocutaneous free flap. Arch Craniofac Surg 2015; 16: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centonze R, Agostini E, Massaccesi S, et al. A novel equine-derived pericardium membrane for dural repair: a preliminary, short-term investigation. Asian J Neurosurg 2016; 11: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seo Y, Kim JW, Dho YS, et al. Evaluation of the safety and effectiveness of an alternative dural substitute using porcine pericardium for duraplasty in a large animal model. J Clin Neurosci 2018; 58: 187–191. [DOI] [PubMed] [Google Scholar]
- 28. Stumpf TR, Sandarage RV, Galuta A, et al. Design and evaluation of a biosynthesized cellulose drug releasing duraplasty. Mater Sci Eng C Mater Biol Appl 2020; 110: 110677. [DOI] [PubMed] [Google Scholar]
- 29. Xu C, Zhao J, Gong Q, et al. Sustained release of vancomycin from bacterial cellulose membrane as dural substitutes for anti-inflammatory wound closure in rabbits. J Biomater Appl 2020; 34: 1470–1478. [DOI] [PubMed] [Google Scholar]
- 30. Sandoval-Sánchez JH, Ramos-Zúñiga R, Luquín De Anda S, et al. A new bilayer chitosan scaffolding as a dural substitute: experimental evaluation. World Neurosurg 2012; 77: 577–582. [DOI] [PubMed] [Google Scholar]
- 31. Zerris VA, James KS, Roberts JB, et al. Repair of the dura mater with processed collagen devices. J Biomed Mater Res B Appl Biomater 2007; 83: 580–588. [DOI] [PubMed] [Google Scholar]
- 32. Esposito F, Cappabianca P, Fusco M, et al. Collagen-only biomatrix as a novel dural substitute. Examination of the efficacy, safety and outcome: clinical experience on a series of 208 patients. Clin Neurol Neurosurg 2008; 110: 343–351. [DOI] [PubMed] [Google Scholar]
- 33. Pettorini BL, Tamburrini G, Massimi L, et al. The use of a reconstituted collagen foil dura mater substitute in paediatric neurosurgical procedures – experience in 47 patients. Br J Neurosurg 2010; 24: 51–54. [DOI] [PubMed] [Google Scholar]
- 34. Kim DW, Eum WS, Jang SH, et al. A transparent artificial dura mater made of silk fibroin as an inhibitor of inflammation in craniotomized rats: Laboratory investigation. J Neurosurg 2011; 114: 485–490. [DOI] [PubMed] [Google Scholar]
- 35. Flanagan KE, Tien LW, Elia R, et al. Development of a sutureless dural substitute from Bombyx mori silk fibroin. J Biomed Mater Res B Appl Biomater 2015; 103: 485–494. [DOI] [PubMed] [Google Scholar]
- 36. Su Y, Li Z, Zhu H, et al. Electrohydrodynamic fabrication of triple-layered polycaprolactone dura mater substitute with antibacterial and enhanced osteogenic capability. Chin JMech Eng Addit Manuf Front 2022; 1: 100026. [Google Scholar]
- 37. Shih TY, Yang JD, Chen JH. Synthesis, characterization and evaluation of segmented polycaprolactone for development of dura substitute. In: Procedia Engineering, Taipei, Taiwan, 19-22 September 2011, pp.144–149. Amsterdam: Elsevier Ltd. [Google Scholar]
- 38. Terasaka S, Taoka T, Kuroda S, et al. Efficacy and safety of non-suture dural closure using a novel dural substitute consisting of polyglycolic acid felt and fibrin glue to prevent cerebrospinal fluid leakage—A non-controlled, open-label, multicenter clinical trial. J Mater Sci Mater Med 2017; 28: 69. [DOI] [PubMed] [Google Scholar]
- 39. Masuda S, Fujibayashi S, Otsuki B, et al. The dural repair using the combination of polyglycolic acid mesh and fibrin glue and postoperative management in spine surgery. J Orthop Sci 2016; 21: 586–590. [DOI] [PubMed] [Google Scholar]
- 40. Kawabata A, Inose H, Ukegawa D, et al. A foreign body granuloma after the usage of polyglycolic acid mesh and fibrin glue for dural repair. A case report. J Orthop Sci 2017; 22: 371–374. [DOI] [PubMed] [Google Scholar]
- 41. Van Doormaal T, Germans MR, Sie M, et al. Single-arm, open-label, multicentre first in human study to e valuate the safety and performa nc e of dur al sealant patch in reducing C S F leakag e following elective cranial surgery: The ENCASE trial. BMJ Open 2021; 11: e049098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li G, Zhao CH, Pu JK, et al. Clinical application of artificial dura mater to avoid cerebrospinal fluid leaks after microvascular decompression surgery. Minim Invasive Neurosurg 2005; 48: 369–372. [DOI] [PubMed] [Google Scholar]
- 43. Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (neuropatch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery 2004; 54: 599–604. [DOI] [PubMed] [Google Scholar]
- 44. Chumnanvej S, Luangwattanawilai T, Rawiwet V, et al. In vivo evaluation of bilayer ORC/PCL composites in a rabbit model for using as a dural substitute. Neurol Res 2020; 42: 879–889. [DOI] [PubMed] [Google Scholar]
- 45. Hemstapat R, Suvannapruk W, Thammarakcharoen F, et al. Performance evaluation of bilayer oxidized regenerated cellulose/poly ε-caprolactone knitted fabric-reinforced composites for dural substitution. Proc Inst Mech Eng H 2020; 234: 854–863. [DOI] [PubMed] [Google Scholar]
- 46. Sanpakitwattana A, Suvannapruk W, Chumnanvej S, et al. Cefazolin loaded oxidized regenerated cellulose/polycaprolactone bilayered composite for use as potential antibacterial dural substitute. Polymers 2022; 14: 4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liao J, Li X, He W, et al. A biomimetic triple-layered biocomposite with effective multifunction for dura repair. Acta Biomater 2021; 130: 248–267. [DOI] [PubMed] [Google Scholar]
- 48. Mack J, Squier W, Eastman JT. Anatomy and development of the meninges: implications for subdural collections and CSF circulation. Pediatr Radiol 2009; 39: 200–210. [DOI] [PubMed] [Google Scholar]
- 49. Nabeshima S, Reese TS, Landis DMD, et al. Junctions in the meninges and marginal glia. J Comp Neurol 1975; 164: 127–169. [DOI] [PubMed] [Google Scholar]
- 50. De Kegel D, Vastmans J, Fehervary H, et al. Biomechanical characterization of human dura mater. J Mech Behav Biomed Mater 2018; 79: 122–134. [DOI] [PubMed] [Google Scholar]
- 51. Ünal M, Sezgin AB. Dura mater: anatomy and clinical implication. J Behav Brain Sci 2021; 11: 239–247. [Google Scholar]
- 52. Borg N, Cutsforth-Gregory J, Oushy S, et al. Anatomy of spinal venous drainage for the neurointerventionalist: from puncture site to intervertebral foramen. Am J Neuroradiol 2022; 43: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. François P, Travers N, Lescanne E, et al. The interperiosteo-dural concept applied to the perisellar compartment: a microanatomical and electron microscopic study. J Neurosurg 2010; 113: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 54. Nagel SJ, Reddy CG, Frizon LA, et al. Spinal dura mater: biophysical characteristics relevant to medical device development. J Med Eng Technol 2018; 42: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinaci A, Bergmann W, Bleys RLAW, et al. Histologic comparison of the dura mater among species. Comp Med 2020; 70: 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Protasoni M, Sangiorgi S, Cividini A, et al. The collagenic architecture of human dura mater: Laboratory investigation. J Neurosurg 2011; 114: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 57. Adeeb N, Mortazavi MM, Tubbs RS, et al. The cranial dura mater: a review of its history, embryology, and anatomy. Child’s Nervous System 2012; 28: 827–837. [DOI] [PubMed] [Google Scholar]
- 58. Nabeshima S, Reese TS, Landis DMD, et al. Junctions in the meninges and marginal glia. J Comparat Neurol 1975; 164: 127–169. [DOI] [PubMed] [Google Scholar]
- 59. Morales-Avalos R, Soto-Domínguez A, García-Juárez J, et al. Characterization and morphological comparison of human dura mater, temporalis fascia, and pericranium for the correct selection of an autograft in duraplasty procedures. Surg Radiol Anat 2017; 39: 29–38. [DOI] [PubMed] [Google Scholar]
- 60. Kapoor C, Vaidya S, Wadhwan V, et al. Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther 2016; 12: 28. [DOI] [PubMed] [Google Scholar]
- 61. Laun A, Tonn JC, Jerusalem C. Comparative study of lyophilized human dura mater and lyophilized bovine pericardium as dural substitutes in neurosurgery. Acta Neurochir 1990; 107: 16–21. [DOI] [PubMed] [Google Scholar]
- 62. Lu KG, Stultz CM. Insight into the degradation of type-I collagen fibrils by MMP-8. J Mol Biol 2013; 425: 1815–1825. [DOI] [PubMed] [Google Scholar]
- 63. Perrini P. Technical nuances of autologous pericranium harvesting for dural closure in chiari malformation surgery. J Neurol Surg B Skull Base 2015; 76: 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kassam A, Carrau RL, Snyderman CH, et al. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19: 1–7. [PubMed] [Google Scholar]
- 65. Snyderman C, Kassam A, Carrau R, et al. endoscopic reconstruction of cranial base defects following endonasal skull base surgery. Skull Base 2007; 17: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. El-Sayed I, Roediger F, Goldberg A, et al. endoscopic reconstruction of skull base defects with the nasal septal flap. Skull Base 2008; 18: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tomita T, Hayashi N, Okabe M, et al. New Dried human amniotic membrane is useful as a substitute for dural repair after skull base surgery. J Neurol Surg B Skull Base 2012; 73: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu L, Dharmadhikari S, Spector BM, et al. Tissue-engineered composite tracheal grafts create mechanically stable and biocompatible airway replacements. J Tissue Eng 2022; 13: 20417314221108791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pereira AR, Trivanović D, Stahlhut P, et al. Preservation of the naïve features of mesenchymal stromal cells in vitro: comparison of cell- and bone-derived decellularized extracellular matrix. J Tissue Eng 2022; 13: 20417314221074453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Islam S, Ogane K, Ohkuma H, et al. Usefulness of acellular dermal graft as a dural substitute in experimental model. Surg Neurol 2004; 61: 297–302. [DOI] [PubMed] [Google Scholar]
- 71. Costantino PD, Wolpoe ME, Govindaraj S, et al. Human dural replacement with acellular dermis: Clinical results and a review of the literature. Head Neck 2000; 22: 765–771. [DOI] [PubMed] [Google Scholar]
- 72. Parlato C, Di Nuzzo G, Luongo M, et al. Use of a collagen biomatrix (TissuDura®) for dura repair: a long-term neuroradiological and neuropathological evaluation. Acta Neurochir 2011; 153: 142–147. [DOI] [PubMed] [Google Scholar]
- 73. Picheth GF, Pirich CL, Sierakowski MR, et al. Bacterial cellulose in biomedical applications: a review. Int J Biol Macromol 2017; 104: 97–106. [DOI] [PubMed] [Google Scholar]
- 74. Rajwade JM, Paknikar KM, Kumbhar J V. Applications of bacterial cellulose and its composites in biomedicine. Appl Microbiol Biotechnol 2015; 99: 2491–2511. [DOI] [PubMed] [Google Scholar]
- 75. Kojima K, Okamoto Y, Kojima K, et al. Effects of chitin and chitosan on collagen synthesis in wound healing. J Vet Medi Sci 2004; 66: 1595–1598. [DOI] [PubMed] [Google Scholar]
- 76. Azad AK, Sermsintham N, Chandrkrachang S, et al. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res 2004; 69B: 216–222. [DOI] [PubMed] [Google Scholar]
- 77. Ramos-Zúñiga R, López-González F, Segura-Durán I. Bilaminar chitosan scaffold for sellar floor repair in transsphenoidal surgery. Front Bioeng Biotechnol 2020; 8: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ueno H, Nakamura F, Murakami M, et al. Evaluation effects of chitosan for the extracellular matrix production by fibroblasts and the growth factors production by macrophages. Biomaterials 2001; 22: 2125–2130. [DOI] [PubMed] [Google Scholar]
- 79. Park CJ, Gabrielson NP, Pack DW, et al. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials 2009; 30: 436–444. [DOI] [PubMed] [Google Scholar]
- 80. Mori T, Murakami M, Okumura M, et al. Mechanism of macrophage activation by chitin derivatives. J Vet Medi Sci 2005; 67: 51–56. [DOI] [PubMed] [Google Scholar]
- 81. Balters L, Reichl S. 3D bioprinting of corneal models: A review of the current state and future outlook. J Tissue Eng 2023; 14: 20417314231197793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Neulen A, Gutenberg A, Takács I, et al. Evaluation of efficacy and biocompatibility of a novel semisynthetic collagen matrix as a dural onlay graft in a large animal model. Acta Neurochir 2011; 153: 2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chang P, Li S, Sun Q, et al. Large full-thickness wounded skin regeneration using 3D-printed elastic scaffold with minimal functional unit of skin. J Tissue Eng 2022; 13: 20417314211063022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stocco E, Porzionato A, De Rose E, et al. Meniscus regeneration by 3D printing technologies: current advances and future perspectives. J Tissue Eng 2022; 13: 20417314211065860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kataoka K, Suzuki Y, Kitada M, et al. Alginate, a bioresorbable material derived from brown seaweed, enhances elongation of amputated axons of spinal cord in infant rats. J Biomed Mater Res 2001; 54: 373–384. [DOI] [PubMed] [Google Scholar]
- 86. Corden TJ, Jones IA, Rudd CD, et al. Initial development into a novel technique for manufacturing a long fibre thermoplastic bioabsorbable composite: in-situ polymerisation of poly-ϵ-caprolactone. Compos Part A Appl Sci Manuf 1999; 30: 737–746. [Google Scholar]
- 87. Bi X, Liu B, Mao Z, et al. Applications of materials for dural reconstruction in pre-clinical and clinical studies: Advantages and drawbacks, efficacy, and selections. Mater Sci Eng C Mater Biol Appl 2020; 117: 111326. [DOI] [PubMed] [Google Scholar]
- 88. Ueda K, Tanaka T, Jinbo M, et al. Sutureless pneumostasis using polyglycolic acid mesh as artificial pleura during video-assisted major pulmonary resection. Ann Thorac Surg 2007; 84: 1858–1861. [DOI] [PubMed] [Google Scholar]
- 89. Hayashibe A, Sakamoto K, Shinbo M, et al. New method for prevention of bile leakage after hepatic resection. J Surg Oncol 2006; 94: 57–60. [DOI] [PubMed] [Google Scholar]
- 90. Deng K, Ye X, Yang Y, et al. Evaluation of efficacy and biocompatibility of a new absorbable synthetic substitute as a dural onlay graft in a large animal model. Neurol Res 2016; 38: 799–808. [DOI] [PubMed] [Google Scholar]
- 91. Shi R, Xue J, Wang H, et al. Fabrication and evaluation of a homogeneous electrospun PCL–gelatin hybrid membrane as an anti-adhesion barrier for craniectomy. J Mater Chem B 2015; 3: 4063–4073. [DOI] [PubMed] [Google Scholar]
- 92. Bartus C, Hanke WC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatologic Surgery 2013; 39: 698–705. [DOI] [PubMed] [Google Scholar]
- 93. Shimada Y, Hongo M, Miyakoshi N, et al. Dural substitute with polyglycolic acid mesh and fibrin glue for dural repair: Technical note and preliminary results. J Orthop Sci 2006; 11: 454–458. [DOI] [PubMed] [Google Scholar]
- 94. Abumanhal M, Ben-Cnaan R, Feldman I, et al. Polyester urethane implants for orbital trapdoor fracture repair in children. J Oral Maxillofac Surg 2019; 77: 126–131. [DOI] [PubMed] [Google Scholar]
- 95. Lewis KM, Sweet J, Wilson ST, et al. Safety and efficacy of a novel, self-adhering dural substitute in a canine supratentorial durotomy model. Neurosurgery 2018; 82: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Park HK, Joo W, Gu BK, et al. Collagen/poly(d,l-lactic-co-glycolic acid) composite fibrous scaffold prepared by independent nozzle control multi-electrospinning apparatus for dura repair. J Ind Eng Chem 2018; 66: 430–437. [Google Scholar]
- 97. Pearcy Q, Tomlinson J, Niestrawska JA, et al. Systematic review and meta-analysis of the biomechanical properties of the human dura mater applicable in computational human head models. Biomech Model Mechanobiol 2022; 21: 755–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grotenhuis JA. Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol 2005; 64: 490–493. [DOI] [PubMed] [Google Scholar]
- 99. Giovanni S, Della Pepa GM, La Rocca G, et al. Galea-pericranium dural closure: can we safely avoid sealants? Clin Neurol Neurosurg 2014; 123: 50–54. [DOI] [PubMed] [Google Scholar]
- 100. Kumar A, Maartens NF, Kaye AH. Evaluation of the use of BioGlue® in neurosurgical procedures. J Clin Neurosci 2003; 10: 661–664. [DOI] [PubMed] [Google Scholar]
- 101. Hutter G, von Felten S, Sailer MH, et al. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomized controlled trial. J Neurosurg 2014; 121: 735–744. [DOI] [PubMed] [Google Scholar]
- 102. Carless PA, Henry DA, Anthony DM. Fibrin sealant use for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev 2003; 2003: CD004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kinaci A, Algra A, Heuts S, et al. Effectiveness of dural sealants in prevention of cerebrospinal fluid leakage after craniotomy: a systematic review. World Neurosurg 2018; 118: 368–376.e1. [DOI] [PubMed] [Google Scholar]
- 104. Preul MC, Bichard WD, Muench TR, et al. Toward optimal tissue sealants for neurosurgery: use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery 2003; 53: 1189–1199. [DOI] [PubMed] [Google Scholar]
- 105. Boogaarts JD, Grotenhuis JA, Bartels RHMA, et al. Use of a novel absorbable hydrogel for augmentation of dural repair: results of a preliminary clinical study. Operat Neurosurg 2005; 57: 146–151. [DOI] [PubMed] [Google Scholar]
- 106. Azzam D, Romiyo P, Nguyen T, et al. Dural repair in cranial surgery is associated with moderate rates of complications with both autologous and nonautologous dural substitutes. World Neurosurg 2018; 113: 244–248. [DOI] [PubMed] [Google Scholar]