Abstract
Background and Aims:
Sepsis-induced immunosuppression appears to be reversible with immunomodulatory drugs. Mycobacterium indicus pranii (MIP) stimulates the Th1 type of immune response. This systematic review and meta-analysis of randomised controlled trials (RCTs) was aimed to find out if MIP is effective at improving clinical outcomes in sepsis patients.
Methods:
The databases (PubMed, Google Scholar, Web of Science, and Cochrane Library), along with preprint servers until June 2023, were searched. The methodology was evaluated using the ‘Cochrane Collaboration risk of bias-2 tool’ for RCT. The study included patients more than 18 years of age with sepsis within 48 h of first organ dysfunction. The primary outcome was 28-day mortality, and secondary outcomes were the length of stay in the intensive care unit (ICU), days on vasopressor support, ventilator-associated pneumonia (VAP), secondary infections, catheter-related bloodstream infections (CRBSI), and the delta sequential organ failure assessment (SOFA) score.
Results:
The meta-analysis included two studies with 252 participants. In a pooled analysis, mortality in the MIP group was 43% lower than in the control (RR: 0.57, 95%CI: 0.33–1); however, this difference was statistically not significant. We observed the days on a vasopressor day (standardised mean difference [SMD]: 0.38; 95%CI: −1.20 to 0.44), length of ICU stay (SMD: 0.46; 95%CI: −1.44 to 0.51), secondary infection (RR: 0.75; 95%CI: 0.19–3.01), VAP (RR: 0.6; 95%CI: 0.28–1.56), CRBSI (RR: 0.97, 95%CI: 0.14–6.98), delta SOFA score (SMD: 0.88, 95%CI: −1.66 to − 0.10) between the two groups.
Conclusions:
Our findings observed preliminary evidence in the trends for a positive association of MIP with better outcomes in sepsis patients.
Keywords: Catheter-related bloodstream infections, mortality, Mycobacterium indicus pranii, meta-analysis, Mycobacterium welchii, sepsis, sequential organ failure assessment score systematic review, vasopressor, ventilator-associated pneumonia
INTRODUCTION
Sepsis is a syndrome caused by a dysregulated host immune response to an infection. Worldwide, sepsis accounts for 49 million incidences and 11 million yearly mortality.[1] In the intensive care unit (ICU), sepsis remains a major and life-threatening issue, with mortality rates ranging from 12% to 40%.[2] Sepsis is prevalent in India at 28.3%, with 20.5% of cases requiring ICU treatment.[3] The dominant response (proinflammatory or anti-inflammatory) is influenced by the host's pre-morbid condition, infectious agent, and prompt administration of therapeutic intervention such as anti-microbials, source control, intravenous fluids, and others.[4,5] Due to the variety of the immune response, numerous treatment strategies addressing a specific inflammatory mechanism in sepsis have been ineffective.[6,7] Immunomodulatory agents, on the other hand, appear to counteract the immunosuppressive state generated by sepsis.[8,9]
Lipopolysaccharide is a component of the cell wall of gram-negative organisms. It interacts with toll-like receptor 4 (TLR4) on host leukocytes and other immune cells such as neutrophils, macrophages, dendritic cells, and natural killer cells. Two important pathways, namely the myeloid differentiation primary response 88 (MyD88) pathway and the toll/interleukin-1 (IL-1) receptor domain-containing adaptor protein-induced interferon (TRIF) pathway, are activated as a result of lipopolysaccharide binding to TLR4. The TRIF pathway produces type-1 interferons, which inhibit the inflammatory response, whereas the MyD88 pathway causes transmission that activates the transcription factors NF-B and activator protein-1.[10] In contrast, the lipoteichoic acid-mediated TLR-2 pathway is stimulated by gram-positive microbes.[11]
Mycobacterium indicus pranii (MIP), earlier called Mycobacteria welchii (Mycobacterium w), is a fast-growing, non-pathogenic atypical mycobacterium. MIP is a heat-killed preparation and has been demonstrated to boost the Th1 type of immune response when delivered intradermally.[12,13]
Immunomodulators have the potential to modulate inflammation in both directions, preventing both immunologic paralysis and excessive inflammation. A few clinical trials with sepsis found that MIP was linked to a significant decrease in mortality, fewer days on mechanical ventilation, reduced hospital and ICU stay duration, lower incidence of nosocomial infection, and reduced delta sequential organ failure assessment (SOFA) score. To ascertain the effectiveness of MIP for enhancing clinical outcomes in sepsis patients, we planned to conduct a systematic review and meta-analysis of randomised controlled trials (RCTs). Our primary outcome was 28-day mortality in patients aged between 18 and 60 years presumed to have sepsis receiving mycobacteria indicus pranii, and secondary outcomes were the length of ICU stay, days on vasopressor support, ventilator-associated pneumonia (VAP), secondary infections, catheter-related bloodstream infections (CRBSI), and the delta sequential organ failure assessment (SOFA) score.
METHODS
Our systematic review was registered prospectively in PROSPERO (CRD42022315378), and the design and method were developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[14] and the Cochrane Handbook for Systematic Reviews of Interventions.[15]
Data source
PubMed, Google Scholar, Web of Science, and Cochrane Library, along with preprint servers such as medRxiv, Research Square, and Social Science Research Network, served as the database for search in this review.
Study selection criteria and types of studies
The search strategy included retrieval of related articles in the above databases from their inception till June 2023. Key terms for searching relevant literature were Mycobacterium indicus pranii OR Mycobacterium welchii OR Mycobacterium w and sepsis and randomised controlled trials (RCTs) as filter [((“mycobacterium intracellulare subsp intracellulare”[Supplementary Concept] OR “mycobacterium intracellulare subsp intracellulare”[All Fields] OR “mycobacterium indicus pranii”[All Fields] OR ((“mycobacteriae”[All Fields] OR “mycobacterias”[All Fields] OR “mycobacterium”[MeSH Terms] OR “mycobacterium”[All Fields] OR “mycobacteria”[All Fields]) AND “welchii”[All Fields]) OR ((“mycobacteriae”[All Fields] OR “mycobacterias”[All Fields] OR “mycobacterium”[MeSH Terms] OR “mycobacterium”[All Fields] OR “mycobacteria”[All Fields]) AND “w”[All Fields])) AND (“sepsis”[MeSH Terms] OR “sepsis”[All Fields])) AND (“randomized controlled trial”[Filter])]. Only RCTs published in English were considered for inclusion, and other study designs such as cohort studies (prospective, retrospective, and cross-sectional studies), abstracts, conference papers, and case series were excluded. Each citation was evaluated twice; disagreements were resolved through conversation and by inviting a third reviewer if necessary. In two phases, two reviewers (JP and KS) separately assessed all potentially significant citations and references, initially looking at titles and abstracts before reading entire publications for those that fit the criteria. Conflicts were resolved by a third reviewer (AK). Studies were chosen that had a well-formulated ‘PICOS’ framework, which included:
Participants - Patients 18 years or older with sepsis presenting within 48 h of first organ dysfunction
Intervention/Exposure - MIP
Comparator/control - Matching placebo
Outcomes - Primary: 28-day mortality, Secondary: length of ICU stay, days on vasopressor, VAP, secondary infections, CRBSI, and delta SOFA score.
Data extraction
A systematic search was performed in databases, and the following characteristics were retrieved from the included studies: sample size, gender, age, ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2 expressed as a fraction) (PaO2/FiO2), haemoglobin, 28-day mortality, days on vasopressor, ICU length of stay, secondary infection, baseline SOFA, delta SOFA, number of patients with VAP, and CRBSI. Two reviewers used a separate data abstraction form to extract data. A two-tiered approach addressed any conflict between and resolved between two reviewers. First, they discussed the issues. Second, if they were still unsettled, we invited a third author to accomplish individual data retrieval, followed by a discussion to settle the dispute.
Assessment of methodological quality
The methodological quality and risk of bias (RoB) were assessed by ‘Cochrane RoB for randomised trials (RoB 2 tool)’.[16] The risk of selection, performance, reporting, detection, attrition, and other biases were assessed and classified as low, high, or unclear risk.
Data synthesis
Direct extraction or indirect computation was used to obtain data. For continuous variables, we used a normal distribution and transformed interquartile ranges to standard deviations (SD) following Cochrane Collaboration guidelines, and we considered the median as the mean for calculation.[17] The current systematic review included studies with adequate quantitative data to compute the effective size.
Statistical analysis
We calculated the pooled effect size using the pooled risk ratio (RR) with a 95% confidence interval (CI). Continuous findings are presented as mean differences (MDs) with 95% CI or standardised mean differences (SMDs) with 95% CI, whereas dichotomous findings are presented as RR with 95% CIs. To pool the data for continuous outcomes with different units, standardised mean difference (SMD) with 95% CI was used, and mean difference (MD) was utilised for the same units. If heterogeneity was low, it was assumed that the true effects of the intervention were the same across all included trials, and a fixed-effect model was used to reflect the best estimate of the intervention effect. However, because of the considerable heterogeneity, we adopted a random-effects model. We evaluated SMD for continuous variables using a random effect model because the effect size for the outcomes is clinically important. The DerSimonian and Laird random-effects models were employed if the degree of heterogeneity was greater than 50%; otherwise, the fixed-effects model was adopted. The study weights were made using the inverse variance method. The Cochran Q test and the I2 statistic were used to evaluate study heterogeneity.[15] For conducting a meta-analysis, we used RevMan version 5.3.5 (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration 2014). A P value of <0.05 was taken as significant.
RESULTS
Of the 21 potentially relevant studies, two studies[11,18] with 252 participants were included in the systematic review [Figure 1]. Table 1 depicts the demographic and clinical characteristics of the included studies. As per RoB assessment, both studies[11,18] had a low bias in terms of random sequence generation, selective reporting, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data; however, allocation concealment and other sources of bias were unclear in one study,[11] and there was a high risk in other source of bias in the other study[18] [Figure 2]. Concerning the primary outcome of the review, the pooled analysis revealed a 43% lower mortality rate in the MIP group than in the control group (RR: 0.57, 95%CI: 0.33–1, I2 = 27%, P = 0.05); days on vasopressor (SMD: 0.38; 95%CI: −1.20 to 0.44, I2 = 85%, P = 0.36); length of ICU stay (SMD: 0.46; 95%CI: −1.44 to 0.51, I2 = 89%, P = 0.35); secondary infection (RR: 0.75; 95%CI: 0.19–3.01, I2 = 86%, P = 0.69); VAP (RR: 0.6; 95%CI: 0.28–1.56, I2 = 52%, P = 0.34); CRBSI (RR: 0.97, 95%CI: 0.14–6.98, I2 = 77%, P = 0.98); and delta SOFA score (SMD: 0.88, 95%CI: −1.66 to − 0.10, I2 = 81%, P = 0.03) [Figure 3] between both the groups.
Figure 1.
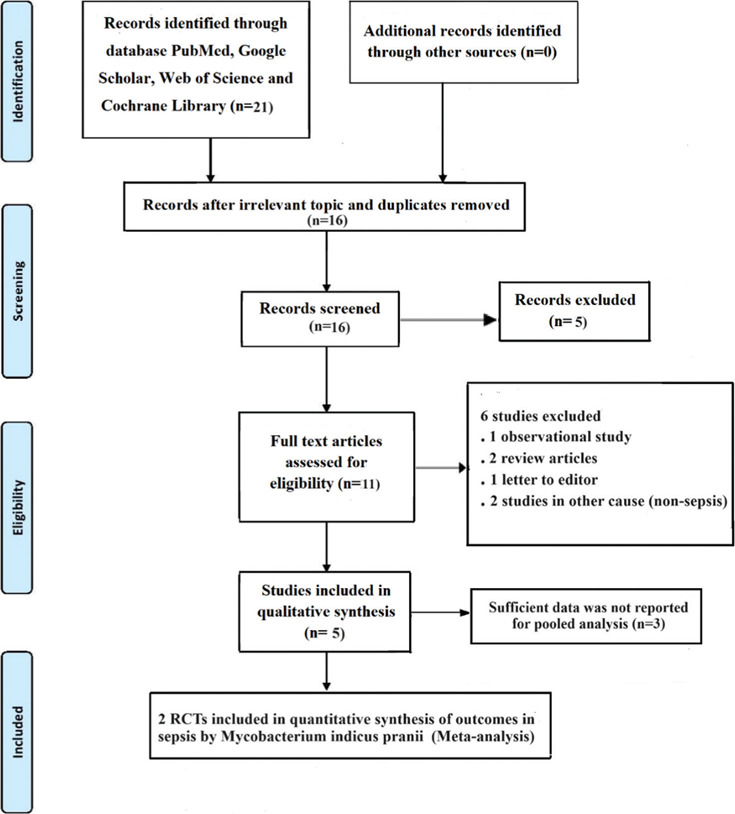
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. RCT = randomised controlled trial
Table 1.
Demographic and clinical characteristics of included studies
| Author | Year | Treatment status | Sample size | Gender |
Age |
PaO2/FiO2 | Hb |
Baseline SOFA |
Delta SOFA |
Mortality | On vasopressor (days) |
ICU length of stay |
Secondary infection | VAP | CRBSI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | Mean | SD | Mean | SD/IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||||||||
| Sehgal et al.[18] [2015] | Intervention | 101 | 29 | 72 | 39.8 | 13.5 | 276 | (174–349) | 9.9 | 2.5 | 7 | 4–10 | 0 | 0–2 | 9 | 1 | 0–2.5 | 8 | 5–14 | 25 | 11 | 10 |
| Control | 100 | 30 | 70 | 40.71 | 13.4 | 226 | (140–349) | 10.6 | 2.4 | 7 | 4–10 | 1 | 0–3 | 20 | 1 | 0–3 | 8 | 4–12.5 | 17 | 11 | 4 | |
| Sehgal et al.[11] [2021] | Intervention | 25 | 14 | 11 | 30 | 17 | 227 | (149–310) | 10.3 | 8.4–11.7 | 11 | 4 | 0 | 0–4 | 7 | 2 | 2–3 | 7 | 5–10 | 5 | 5 | 2 |
| Control | 25 | 14 | 11 | 40 | 21.5 | 194 | (115–295) | 11.5 | 9.8–13.5 | 9 | 4 | 4 | 0.5–4.5 | 8 | 3 | 2–4 | 12 | 9–171 | 14 | 12 | 6 | |
Data are presented as n(%), mean (standard deviation) or median (interquartile range). Hb - Haemoglobin, PaO2/FiO2 - ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen, SOFA - Sequential Organ Failure Assessment, VAP - Ventilator-associated pneumonia, CRBSI - Catheter-related bloodstream infection, M- Male, F- Female, IQR - Interquartile range, SD - Standard deviation, ICU - Intensive care unit
Figure 2.
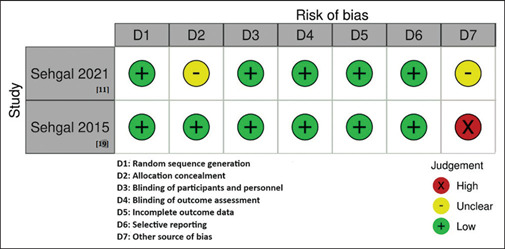
Risk of Bias Summary
Figure 3.
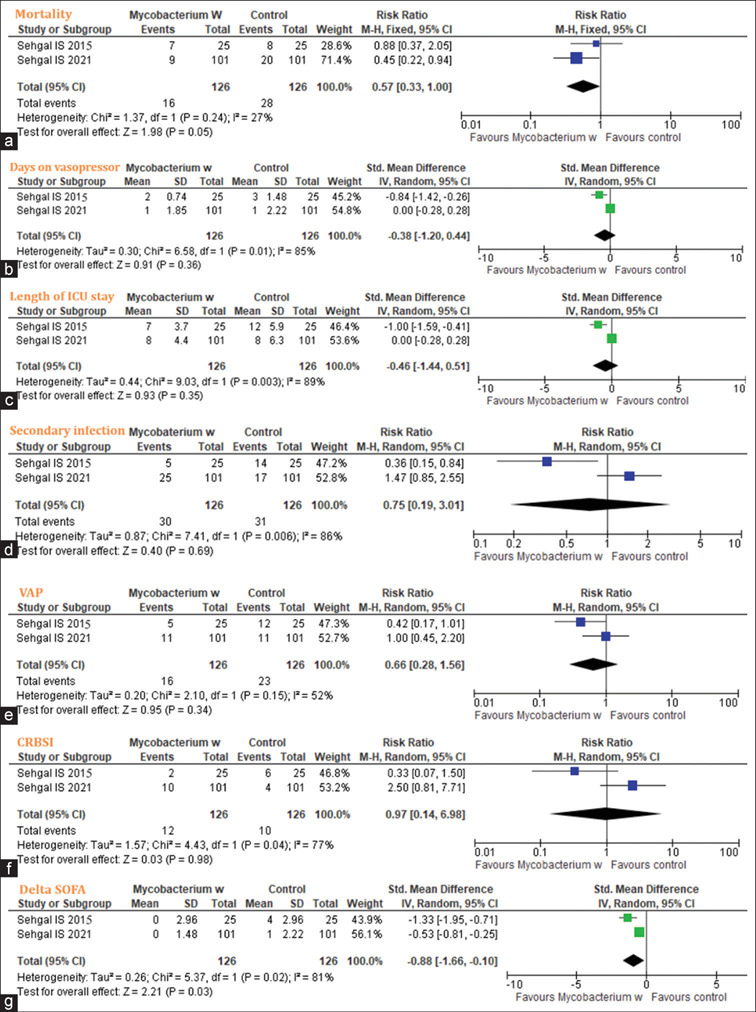
Forest plot showing mortality (a); days on vasopressor (b), length of intensive care unit (ICU) stay (c), secondary infection (d), ventilator-associated pneumonia (e), catheter-related bloodstream infections (f), and delta sequential organ failure assessment (SOFA) (g). CI: confidence interval, M–H: Mantel–Haenszel
DISCUSSION
Our study's analysis, which considered RCT, indicates that MIP plays a role in sepsis. When combined with standard therapy in severe sepsis, we observed that MIP did not affect the 28-day mortality rate. However, the time spent in the ICU and hospital using a mechanical ventilator and the prevalence of secondary infections decreased significantly in earlier studies.[11] Another trial observed that using Mycobacterium w or MIP and standard therapy decreased the 28-day mortality in severe sepsis brought on by probable gram-negative bacteria. However, there was no discernible difference in the number of days without a ventilator, days requiring a vasopressor, and days spent in the ICU or hospital.[18]
In this meta-analysis, we observed a trend in the reduction of death in the MIP group compared to control. However, statistical differences could not be observed. The meta-analysis involved only two studies, limiting the power to observe any statistically significant difference. The days on vasopressor in the MIP group compared to control was noted with 85% heterogeneity. The pooled length of ICU stay was also lower in the intervention group compared to the control but statistically non-significant, with a heterogeneity of 89%. The MIP group also showed lower secondary infection with high heterogeneity (86.5%). High heterogeneities were also observed for VAP and CRBSI (>50%). However, for the delta SOFA, the statistically non-significant trend was observed in favour of the MIP group compared to the control with heterogeneity (81%). These findings indicate conducting future RCT to determine MIP's precise role in sepsis patients.
It was found that patients with severe sepsis who received MIP in addition to standard therapy did not experience a reduction in 28-day mortality. However, there was a significant reduction in the number of days spent in the ICU and hospital, the number of days on a mechanical ventilator, the frequency of secondary bacterial infections, and the delta SOFA score.[18] However, there was no difference in ventilator-free days, days of vasopressor therapy, and ICU or hospital length of stay, and the overall mortality in another study was 14%.[11]
Many studies are ongoing, considering the importance of the needed research in this area. Two studies in the clinical trial registry have been completed, and their results are awaited. The findings of these studies would be significant in determining the precise role of MIP in sepsis patients. The heterogeneity exploration was not possible due to an inadequate number of studies. Publication bias could not be examined due to the non-availability of a sufficient number of studies.
The limitations were the small number of included studies, two in number, and a high degree of heterogeneity among the studies, highlighting the importance of conducting well-designed RCT.
CONCLUSION
Our meta-analysis findings observed preliminary evidence in the trends for a positive association of MIP with better outcomes in sepsis patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to express our gratitude to Prof (Dr) Pradip Kumar Bhattacharya, HOD, Critical Care, RIMS, Ranchi, who supported us in this study. This paper won the 2nd prize in the ‘Best Paper Award’ category in the 3rd World Conference on Comprehensive Critical Care (W4C) at Jaipur in 2023.
REFERENCES
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet. 2020;395:200–11. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Ñamendys-Silva SA, et al. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum Infect Dis. 2018;5:313. doi: 10.1093/ofid/ofy313. doi: 10.1093/ofid/ofy313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee S, Bhattacharya M, Todi SK. Epidemiology of adult-population sepsis in India: A single centre five year experience. Indian J Crit Care Med. 2017;21:573–7. doi: 10.4103/ijccm.IJCCM_240_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, et al. Effect of eritoran, an antagonist of MD2- TLR4, on mortality in patients with severe sepsis: The ACCESS randomised trial. JAMA. 2013;309:1154–62. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 7.Lv S, Han M, Yi R, Kwon S, Dai C, Wang R. Anti-TNF-alpha therapy for patients with sepsis: A systematic meta-analysis. Int J Clin Pract. 2014;68:520–8. doi: 10.1111/ijcp.12382. [DOI] [PubMed] [Google Scholar]
- 8.Bo L, Wang F, Zhu J, Li J, Deng X. Granulocyte-colony stimulating factor (GCSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: A meta-analysis. Crit Care. 2011;15:R58. doi: 10.1186/cc10031. doi: 10.1186/cc10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, et al. Reversal of immunoparalysis in humans in vivo: A double-blind, placebo-controlled, randomised pilot study. Am J Respir Crit Care Med. 2012;186:838–45. doi: 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 10.Pandey RK, Sodhi A, Biswas SK, Dahiya Y, Dhillon MK. Mycobacterium indicus pranii mediates macrophage activation through TLR2 and NOD2 in a MyD88 dependent manner. Vaccine. 2012;30:5748–54. doi: 10.1016/j.vaccine.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Sehgal IS, Basumatary NM, Dhooria S, Prasad KT, Muthu V, Aggarwal AN, et al. A randomised trial of mycobacterium w in severe presumed gram-negative sepsis. Chest. 2021;160:1282–91. doi: 10.1016/j.chest.2021.03.062. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SK, Katoch K, Sarin R, Balambal R, Kumar Jain N, Patel N, et al. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in category II pulmonary tuberculosis in a randomised trial. Sci Rep. 2017;7:3354. doi: 10.1038/s41598-017-03514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayosi BM, Ntsekhe M, Bosch J, Pandie S, Jung H, Gumedze F, et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014;371:1121–30. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. The Cochrane Collaboration. Oxford: 2011. [Last assessed on 2023 Aug]. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Available from: http://handbook-5-1.cochrane.org . [Google Scholar]
- 16.Higgins JPT, Savović J, Page M, Elbers R, Sterne JAC. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd. Chichester UK: Wiley-Blackwell; 2019. pp. 205–228. [Google Scholar]
- 17.Higgins JPT, Li T, Deeks JJ. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2019. Choosing effect measures and computing estimates of effect; pp. 143–176. [Google Scholar]
- 18.Sehgal IS, Agarwal R, Aggarwal AN, Jindal SK. A randomised trial of Mycobacterium w in severe sepsis. J Crit Care. 2015;30:85–8. doi: 10.1016/j.jcrc.2014.08.012. [DOI] [PubMed] [Google Scholar]


