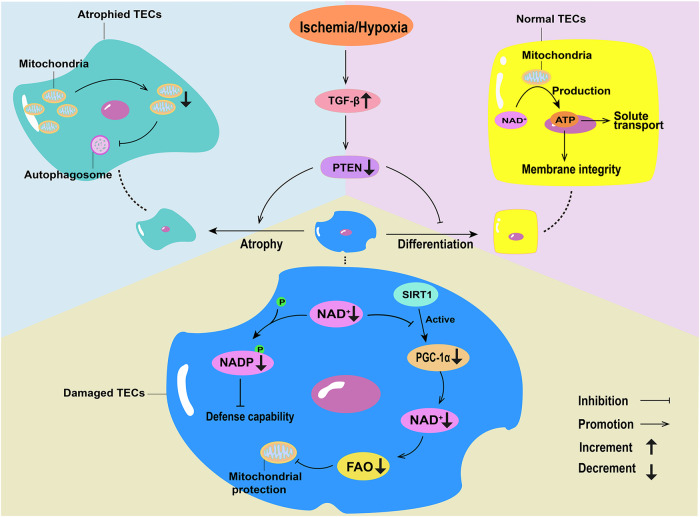
Fig. 1. Changes in mitochondrial energy metabolism in ischemia-induced acute kidney injury (AKI).
Hypoxia can trigger TGF-β, and the increase in TGF-β leads to PTEN defects in renal tubules, inducing tubule atrophy, reducing mitochondrial mass, and causing abnormal autophagy. In healthy tubular epithelial cells (TECs), NAD+ produces ATP through the mitochondria, supplying solute transport and maintaining membrane integrity. In normal TECs, SIRT1 activates PGC1α through the de novo pathway, and promotes the production of NAD+, thereby enhancing fatty acid oxidation (FAO) and mitochondrial protection. In damaged TECs, the consumption of NAD+ inhibits PGC1α, reduces the production of NAD+, decreases FAO, and weakens the protective effect on mitochondria. The depletion of NAD+ inhibits the phosphorylation of NADP, and decreases the defense ability of TECs against ischemia-reperfusion injury-induced oxidative stress.