Abstract
Background
Brentuximab Vedotin (BV) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) is approved in the upfront setting for advanced stage classical Hodgkin lymphoma (cHL). HIV infected patients were excluded from these studies. We aimed to understand the activity and safety of BV-AVD in people living with HIV diagnosed with Hodgkin lymphoma, while focusing on HIV disease parameters and anti-retroviral therapy (ART) interactions.
Methods
We present the phase II portion of a multicenter phase I/II study. Eligible patients were ≥ 18 years of age, had untreated stage II-IV HIV-associated classical HL (HIV-cHL), karnofsky performance status (KPS) > 30%, a CD4 + T-cell count ≥ 50 cells/μl, were required to take ART, and were not on strong CYP3A4/P-glycoprotein inhibitors. Patients were treated with 1.2 mg/kg of BV, defined previously as the recommended phase 2 dose, with standard doses of AVD for 6 cycles on days 1 and 15 of a 28-day cycle. The primary endpoint of the phase 2 portion was 2-year progression-free survival (PFS). All analysis were done per protocol and accrual has been completed. (ClinicalTrials.gov # NCT01771107).
Findings
Between March 8, 2013 to March 7, 2019 41 patients received study therapy with a median follow up of 29 months (IQR 16–38 mos). Thirty-four patients (83%) presented with stage III/IV and 7 (17%) with stage II unfavorable HIV-cHL. Of those who completed therapy, 37/37, (100%) achieved a CR. The 2-year PFS and OS were 86% (95% CI: 0.71, 0.94) and 92% (95% CI: 0.78,0.97), respectively. The most common adverse events grade (G) ≥3 were peripheral sensory neuropathy 10% (4 of 41), neutropenia 44% (18 of 41), and 12% febrile neutropenia, 5 of 41 patients. One treatment related death was reported, due to infection. CD4+/CD8+ T-cell counts increased in 81% of patients during therapy. Baseline CD4+ T-cell counts were below 200 (32–179) cells/μl in 15 patients, but increased above 200 (254–530) cells/μl in 87% after 28 days. Analysis of ABVD treated HIV-cHL historical data demonstrated decreases in CD4+/CD8+ T-cell counts during therapy.
Interpretation
BV-AVD is safe and highly active in HIV-cHL and is an important therapeutic option for HIV-cHL. The CR rate is encouraging and is possibly related to a unique aspect to HIV-cHL biology. Upcoming 5 year data will evaluate the sustainability of the outcomes obtained.
Introduction
People living with HIV (PLWH) have a 6–15-fold increase risk of being diagnosed with classical Hodgkin lymphoma (HIV-cHL) above that of the general population.1,2 HIV-cHL remains one of the most common non-AIDS defining malignancies, most often presenting with CD4+ T-cell counts above 200 cells/μl, advanced stage, and higher international prognostic scores (IPS) than the non-HIV population.4,5 Outcomes have improved since the introduction of combined anti-retroviral therapy (ART) in the mid 1990’s, elevating the overall survival (OS) utilizing doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) from 48% at 2 years to 75–85% at 5 years for advanced stage disease, similar to the non-HIV population.6–8 While outcomes have improved with the addition of ART to HIV-cHL therapy, advanced stage disease continues to have a rate of relapse of 30%, necessitating the need for newer therapies.9
Brentuximab vedotin (BV), is an anti-CD30 antibody drug conjugate coupled to mono-methyl auristatin E (MMAE). It is FDA approved for three indications for Hodgkin lymphoma: as a single agent in relapsed cHL, consolidation post autologous stem cell transplant and as first line treatment in advanced stage cHL, BV in combination with AVD.10,11 In the last setting, BV-AVD showed superior modified progression free survival (PFS) of 82% vs. 77% over the standard ABVD, in the phase III ECHELON-1 study.11 An updated efficacy analysis confirmed the PFS and described an overall survival (OS) advantage of 94% BV-AVD vs. 89 % ABVD respectively at 6 years.12 Patients presenting with elevated IPI scores and stage IV disease particularly benefited, making this therapy particularly relevant in the HIV setting.11,12 As HIV patients were excluded from the ECHELON-1 trial, the AIDS Malignancy Consortium (AMC) in collaboration with the Lymphoma Study Association (LYSA) designed AMC 085, a single arm trial for the upfront treatment of stage II-IV HIV-cHL, with BV-AVD. Here we report the phase II results with unexpected concomitant rise of CD4/8+ T-cell counts.
Methods
Study design and participants
This was an open label phase II study. All sites had institutional review board approval and patients provided written consent. All research was done in accordance with the precepts of the Helsinki declaration. The study protocol utilized in both the United States and France is available in the appendix p15–242. Patients were enrolled in the AMC in the United States and in the LYSA in France. BV was given sequentially just after AVD on days 1 and 15 on a 28-day schedule, for 6 cycles as previously reported.11–13 The recommended phase 2 dose (RP2D) determined in the phase 1 portion of the study was 1.2 mg/kg.13 Per protocol, if all the phase 1 participants received the RP2D, subjects in the phase 1 and 2 portion could be combined for analysis.
Only patients with untreated CD30-positive HIV-cHL stage II–IV were eligible for study. In France only stage III/IV subjects were enrolled. Diagnoses were confirmed by central pathology review and HIV by enzyme-linked immunosorbent assay (ELISA) or an HIV viral load of over 1000 copies/ml. Other key eligibility requirements included ≥ 18 years of age, and a Karnofsky Performance Status (KPS) > 30%. All patients were required to be on ART, though strong CYP3A4/P-glycoprotein inhibitors were excluded. Patients had to change to a non-zidovudine, non-ritonavir, and non-cobicistat-based HIV regimen 7 days prior to therapy. Further ART utilization guidelines are described in appendix p10. Abbreviated inclusion criteria included: CD4+ T-cell count ≥ 50 cells/μl, serum creatinine of ≤ 1.5 mg/dl, absolute neutrophil count ≥1000 cells/μl, and AST and ALT ≤ 3 times the upper limit of normal (ULN), cardiac ejection fraction ≥ 50%, and bilirubin ≤ 1.5 times the ULN unless attributed to atazanavir, in which case the total bilirubin could be ≤ 3.5. All patients with exposure to hepatitis B, C, or evidence of chronic infection with or without immunity to hepatitis B were permitted on study provided patients were taking anti-hepatitis therapy and fulfilled hepatic function criteria. All patients with acute hepatitis B, or with normal transaminases but HBsAg+ or hepatitis core antigen IgM+ were excluded.
Procedures
Subjects with previously untreated HIV-associated classical HL (HIV-cHL) and a CD4 + T-cell count ≥ 50 cells/μl were treated with 1.2 mg/kg BV with standard doses of AVD ( doxorubicin (25 mg/m2), vinblastine (6 mg/m2), and Dacarbazine (375 mg/m2) for 6 cycles on days 1 and 15 of a 28-day cycle. Dose reductions and modifications are described in more detail in appendix p6–9 and in the protocol, appendix p15. Prophylaxis for pneumocystis jiroveci was required and granulocyte colony stimulating growth factor was administered with each cycle per protocol. Quinolone antibacterial prophylaxis was recommended for patients with CD4+ T-cell counts below 100 or whose CD4+T-cell count were expected to fall below 100 cells/μl. The use of strong CYP 3A4 and p-glycoprotein inhibitors were excluded from study and were managed according to appendix p10.
Baseline assessments included evaluation of disease-related signs and symptoms, physical examination, and bone marrow biopsy, CD4+ and CD8+ T-cell counts, HIV-1 RNA viral load (VL), sedimentation rate, and a multigated acquisition scan (MUGA) or echocardiogram. Imaging included computer tomography (CT) of the neck, chest abdomen, and pelvis and positron emission tomography (PET) at baseline, after cycle 2, and at treatment completion. Treatment evaluations included a history and physical, documentation of ART compliance, complete blood count (CBC) and serum chemistries prior to each cycle. CD4+ and CD8+ T-cell counts, HIV-1 RNA VL analysis was performed at the start of cycle 2, 5, end of treatment, and every 3 months afterwards for 2 years. All adverse events (AEs) were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0. CT scans were also done after study completion every 6 months for 5 years. Bone marrows showing HIV-cHL were repeated to confirm complete remission. Staging and response evaluation followed the 2007 Cheson criteria.14 PET/CT evaluation followed the Deauville Criteria, where a score ≤ 3 was considered to be complete radiographic remission.14
Outcomes
The primary endpoint was 2-year progression free survival (PFS) defined as time to progression of disease or death. No subject received additional chemotherapy or consolidative radio-therapy in the absence of progression. Secondary endpoints were to estimate the partial response (PR) rate, complete response (CR) rate, and overall survival (OS) at 2 and 5 years; to evaluate the toxicity of BV-AVD with concurrent ART; and the effect of BV-AVD on CD4, CD8+ T-cell counts and viral loads after cycle 1, 4, at the end of therapy, and every 3 months after treatment completion for one year. To investigate the prognostic value of FDG-PET/CT scans at baseline, after cycle 2, and at treatment completion, with respect to 2-year progression free survival. The recommended phase 2 dose of brentuximab vedotin in combination with AVD was the primary outcome measured in the phase 1 portion of the study, and determined the dose utilized in the phase 2 portion presented.
Exploratory objectives were planned to correlate EBV-associated tumor derived DNA in the serum and cytokine profile during therapy to understand there prognostic significance; and latent and expressed HIV reservoirs, before during and after therapy. These objectives are not reported in this manuscript as they have not been completed to date either due to the few PFS or OS events limiting our ability to draw conclusions with respect to prognosis, or due to technical complications resulting in delays in analysis. Details of the secondary and exploratory endpoints can be found in appendix p2.
Statistical analysis
The primary objective of the study was to obtain an estimate of two-year progression free survival (PFS). The sample size calculation of 51 participants was derived from the hypothesis that the 2- year PFS was 85%. with a 95% confidence interval ±10%. The Kaplan-Meier method was used to estimate PFS and overall survival (OS). The standard error for corresponding 95% confidence interval was computed using Greenwood’s formula. Patients who were progression-free and alive or with unknown status were censored at the date of last contact. Descriptive statistics were reported as median and range for continuous variables; and as frequency (percentage) for categorical variables. Descriptive summaries and exploratory analyses for secondary objectives were conducted. Significance for comparisons were at the p<0.05 level. One post-hoc analysis was performed, analysis of the median CD4 and 8+ T-cell counts at baseline, during therapy and post therapy of 2 cohorts with undetectable viral loads treated with ABVD utilized as a historical control (appendix p11,12), the analysis was descriptive. Statistical analysis were performed using the Statistical Analysis System (SAS) version 9.4.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Results
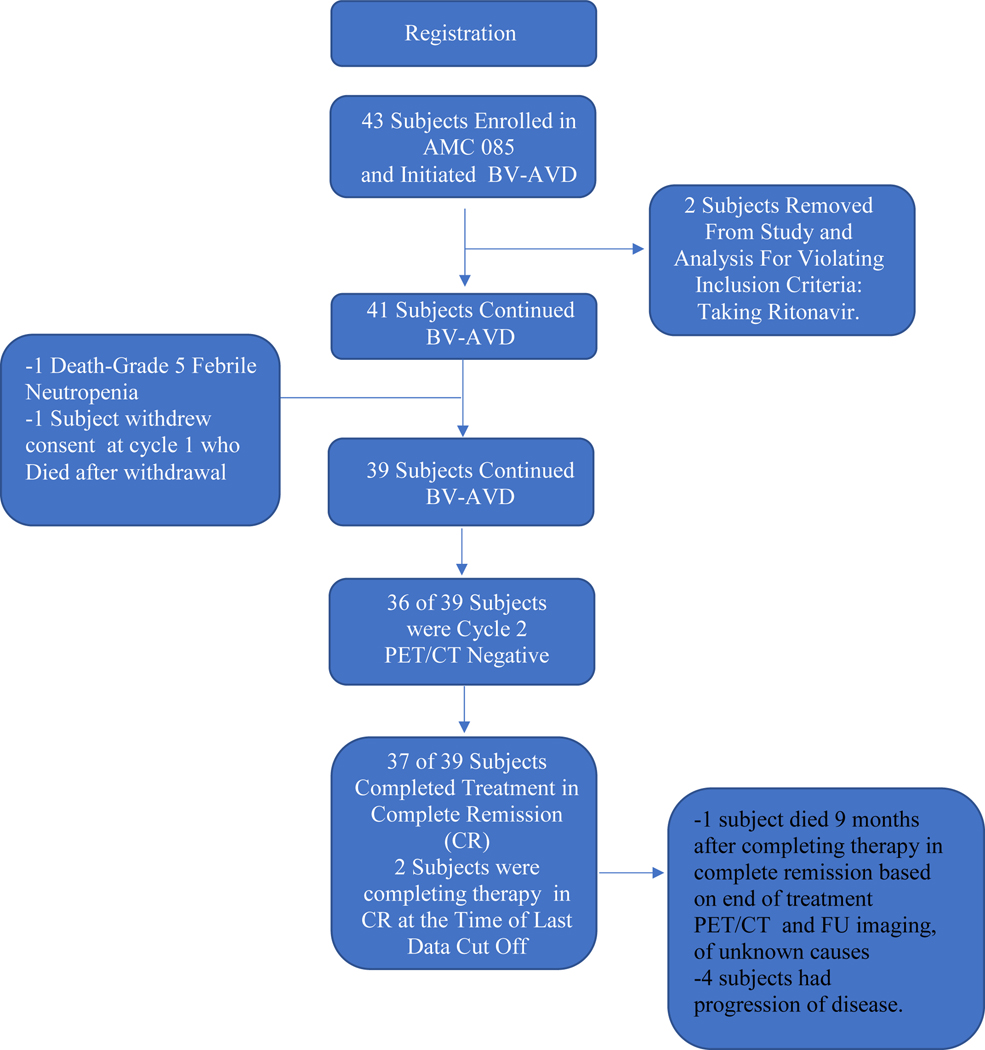
Forty-one patients with previously untreated stage II-IV HIV-cHL were recruited from March 8, 2013 to March 7, 2019. The study was terminated earlier than planned in light of the favorable outcomes and concern that additional patient accrual would only delay dissemination of the results (Figure 1).
Figure 1. Trial Profile.
Patient’s HIV and lymphoma characteristics at baseline are described in Table 1. The median age was 48 (IQR:19), and 93% of the patients were male. The median age of patients enrolled in France was 52, 15 years older than those in the United Sates. Only 10% of the patient’s in France had CD4+ T-cell counts below 200 cells/μl, compared to 50% of those enrolled in the United States. Seventeen percent had stage II (n=7), 27% stage III (n=11), and 56% (n=23) stage IV HIV-cHL. All 7 stage II patients had unfavorable characteristics. Six of 7 stage II patients presented with sedimentation rates >30 and B symptoms. Three of 7 patients had more than 3 nodal areas of involvement. Among patients with advanced stage cHL, 67% had stage IV at presentation, and 75% had IPI scores 3≥. cHL subtypes were nodular sclerosis (41%), mixed cellularity (36%), lymphocyte depleted (2%) and 20% cHL unclassifiable (Table 1).
Table 1:
Patient Demographic and Clinic Characteristics at Baseline
| Variable | Overall (n=41) | AMC (N=32) | LYSA (N=9) |
|---|---|---|---|
| Age (Years) | |||
| Median | 48 | 37 | 52 |
| Range (Min,Max) | (24,67) | (24,67) | (41,61) |
| Gender- No. (%) | |||
| Male Sex | 38 (93) | 29 (91) | 9 (100) |
| Ethnicity- No. (%) | |||
| Hispanic or Latino (%) | 9 (22) | 9 (28) | |
| Not Hispanic or Latino (%) | 21 (51) | 21 (66) | |
| Unknown (%)1 | 11 (27) | 11 (6) | 9 (100)1 |
| Race- No. (%) | |||
| White (%) | 18 (44) | 18 (56) | |
| Black (%) | 11 (27) | 11 (34) | |
| Unknown (%) | 12 (29) | 3 (9) | 9 (100) |
| Histologic Subtype of cHL- No. (%) | |||
| Mixed Cellularity | 15 (36) | 9 (28) | 6 (67) |
| Nodular Sclerosis | 17 (41) | 15 (47) | 2 (22) |
| Lymphocyte Deplete | 1 (2) | 1 (3) | NA |
| Not Otherwise Specified (NOS) | 8(20) | 7 (22) | 1 (11) |
| Ann Arbor Stage- No. (%) | |||
| II favorable | 0 (0) | 0 (0) | 0 (0) |
| II Unfavorable | 7 (17) | 7 (22) | |
| III | 11 (27) | 8 (25) | 3 (33) |
| IV | 23 (56) | 17 (53) | 6 (66) |
| IPS (N=33)- No. (%) | |||
| 0–2 | 9 (27) | 7 (29) | 2 (22) |
| 3–4 | 15(46) | 10 (42) | 5 (56) |
| 5–7 | 9 (27) | 7 (29) | 2 (22) |
| Stage II- No. (%) | |||
| Unfavorable | 7 (100) | 7 (100) | |
| ESR >30 | 6 (86) | 6 (86) | |
| >3 Nodal Areas Involved | 3 (43) | 3 (43) | |
| Baseline CD4+ T-cell Count at Dx-No. (%) | |||
| Median (Range) # CD4+ T-cells cells/μl | 258 (32,818) | 202.5 (32,818) | 310 (105,692) |
| >200 cells/ul No. (%) | 24 (59) | 16 (50) | 8 (89) |
| <200 cells/ul No. (%) | 17 (41) | 16 (50) | 1 (11) |
| Median (Range) # CD8+ T-cells cells/μl | 405 (26,1780) | 386 (26,1780) | 559 (130,1106) |
| Baseline HIV-1 Viral Load-No. (%) | |||
| Median (Range) # of Copies/ml | 40 (22,15706) | ||
| Patients with Detectable Viral Load2 | 9 (22)2 | ||
| CDC HIV Risk Group (N = 31) | |||
| Homosexual/Bisexual: Yes | 17 (55) | 17 (55) | |
| Heterosexual: Yes | 11 (35) | 11 (35) | |
| IV Drug User: Yes | 0 (0) | 0 (100) | |
| Transfusion Recipient: Yes | 1 (3) | 1 (3) | |
| Hemophiliac: Yes | 0 (0) | 0 (0) | |
| Other: Yes | 1 (3) | 1 (3) | |
| Unknown (%)1 | 11 (27) | 2 (6) | 9 (100)1 |
| Smoking History | |||
| Non-smoker | 13 (32) | 13 (41) | 0 (0) |
| Current smoker | 20 (49) | 12 (38) | 8 (89) |
| Former smoker | 8 (20) | 7 (22) | 1 (11) |
Note: For IPS categories, N = 24 and 9 for United States and European sites respectively. For CDC HIV risk group, the description for other category includes – “contracted at birth”.
Subjects enrolled by LYSA could not be asked details of Ethnicity and/or CDC risk group per guidelines.
Only 9 subjects had a positive viral load at baseline, thus the viral load data was combined as a whole, and not separated by LYSA and AMC cohorts.
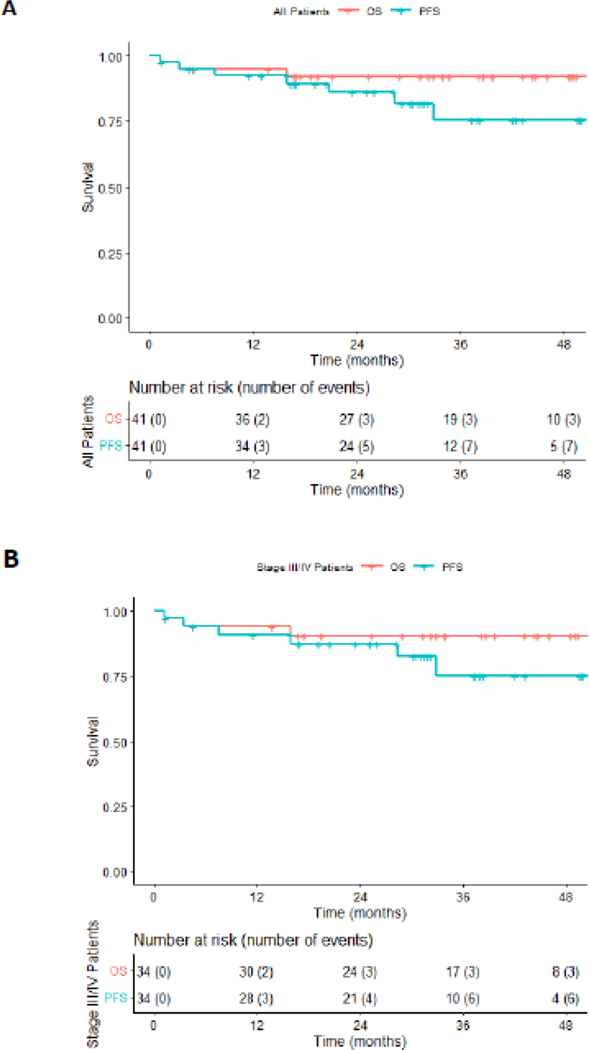
The two year PFS, was 87% (95% CI: 0.71, 0.94) with an OS of 92% (95% CI: 0.78,0.97) for the entire cohort (Figure 2A). For advanced stage HIV-cHL only, the 2 year PFS was 87% (95% CI: 0.71, 0.94) and with an OS of 90%, (95% CI:0.74,0.97) (Figure 2B). Four subjects had progression of disease by the last data cut-off. The only treatment related death was due to febrile neutropenia in cycle 1 and another patient withdrew from the study, declining treatment after cycle 1, and died of infectious complications 1 month after withdrawal. Off treatment, one subject died of unknown causes, 9 months after therapy, in complete remission based on end of treatment PET/CT and follow up imaging (Figure 1). Thirty-six of 39 patients (92%) had a negative interim PET/CT scan after cycle 2 (Deauville score of ≤ 3). All 3 patients with positive-interim PET scans were in remission at the end of therapy. One patient had a positive PET/CT scan post therapy, but had a negative biopsy of the PET avid area and no evidence of malignancy on follow-up. All patients (37) who completed therapy achieved a complete response (CR). The latest assessment was performed after a median follow-up of 29 months (IQR 16–38 mos). At the time of data cut off, 2 subjects had completed post cycle 2 PET/CT scans showing complete remission, but had not completed therapy (Figure 1).
Figure 2. Progression Free (PFS) and Overall Survival (OS) Analysis.
(A) Kaplan-Meier curves showing PFS and OS for the entire cohort and (B) for patients with stage III/IV HIV-associated cHL.
Table 2 presents all G1 and 2 AE reported in over 10% of the patients along with all G3, G4, and G5 AEs reported. Appendix p3 and 4 provides a breakdown all AE by individual grade and by site. The most common non-hematological AE was all grade (G) peripheral sensory neuropathy (PN) at 48% with 10% G3/4 PN, thus mostly G1/2, and managed with dose delays or reductions (appendix p7). G3/4 neutropenia was 42% (18 of 41 patient), the most common hematologic toxicity identified, and was manageable per dose modifications utilized (appendix p6). Febrile neutropenia was identified in 12% (5 of 41 patient). Only one treatment related death was reported, due to infection, and one post treatment death occurred while in CR, of unknown causes. For comparison purposes, the percent of major hematological and non-hematological AE noted in the ECHELON-1 study compared to the current study is shown is appendix p5. As described above, one patient withdrew from the study, declining treatment, and died of infectious complications 1 month after withdrawal. A total of twelve patients had dose reductions and or delays per protocol of either BV and/or AVD, additionally, 3 patients had to discontinue therapy, due to toxicity.
Table 2:
Hematological and non-Hematological Grade I/II AE observed in >10% of Patients and All Grade 3,4,and 5 AE Reported.
| Non-Hematological AE | |||||
|---|---|---|---|---|---|
| Grade | Grade 1 N (%) | Grade 2 N(%) | Grade 3 N(%) | Grade 4 N(%) | Grade 5 N(%) |
| AE Term | |||||
| Abdominal Pain | 2(5) | 5(12) | 1(2) | 0(0) | 0(0) |
| Constipation | 11(26) | 2(5) | 1(2) | 0(0) | 0(0) |
| Diarrhea | 6(14) | 2(5) | 5(12) | 0(0) | 0(0) |
| Nausea | 9(21) | 4(9) | 0(0) | 0(0) | 0(0) |
| Vomiting | 5(12) | 0(0) | 1(2) | 0(0) | 0(0) |
| Fatigue | 8(19) | 5(12) | 0(0) | 0(0) | 0(0) |
| Pain | 6(14) | 2(5) | 1(2) | 0(0) | 0(0) |
| Catheter Related Infection | 0(0) | 0(0) | 2(5) | 0(0) | 0(0) |
| Hepatic Infection | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) |
| Lung Infection | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) |
| Neurosyphilis, ocular | 0(0) | 0(0) | 0(0) | 1(2) | 0(0) |
| Sepsis | 0(0) | 0(0) | 0(0) | 1(2) | 2(2) |
| Soft tissue infection | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) |
| ALT Increased | 6(14) | 2(5) | 0(0) | 0(0) | 0(0) |
| ALK Phosphatase Increased | 10(23) | 0(0) | 0(0) | 0(0) | 0(0) |
| AST Increased | 7(16) | 0(0) | 1(2) | 0(0) | 0(0) |
| Bilirubin Increased | 1(2) | 1(2) | 1(2) | 0(0) | 0(0) |
| CO Diffusion Capacity Dec. | 1(2) | 1(2) | 1(2) | 0(0) | 0(0) |
| Anorexia | 2(5) | 1(2) | 1(2) | 0(0) | 0(0) |
| Hyperglycemia | 5(12) | 0(0) | 1(2) | 0(0) | 0(0) |
| Hypoglycemia | 4(9) | 1(2) | 0(0) | 0(0) | 0(0) |
| Hypokalemia | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) |
| Hyponatremia | 2(5) | 0(0) | 2(5) | 0(0) | 0(0) |
| Hypophosphatemia | 0(0) | 1(2) | 1(2) | 0(0) | 0(0) |
| Back Pain | 4(9) | 1(2) | 0(0) | 0(0) | 0(0) |
| Bone Pain | 2(5) | 3(7) | 0(0) | 0(0) | 0(0) |
| Depressed Level of Consciousness | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) |
| Headache | 5(12) | 0(0) | 0(0) | NA | NA |
| Peripheral Sensory Neuropathy | 9(21) | 7(16) | 4(9) | 0(0) | 0(0) |
| Peripheral Motor Neuropathy | 1(2) | 3(7) | 2(5) | 0(0) | 0(0) |
| Anxiety | 5(12) | 1(2) | 0(0) | 0(0) | 0(0) |
| Cough | 5(12) | 1(2) | 0(0) | 0(0) | 0(0) |
| Dyspnea | 5(12) | 0(0) | 1(2) | 0(0) | 0(0) |
| Hypoxia | 0(0) | 0(0) | 1(2) | 0(0) | 0(0) |
| Alopecia | 4(9) | 4(9) | 0(0) | 0(0) | 0(0) |
| Rash Maculopapular | 5(12) | 2(5) | 0(0) | 0(0) | 0(0) |
| Hematological AE | |||||
| Grade | Grade 1 N (%) | Grade 2 N (%) | Grade 3 N (%) | Grade 4 N(%) | Grade 5 N(%) |
| AE Term | |||||
| Anemia | 4 (9) | 6 (14) | 9(21) | 0(0) | 0(0) |
| Febrile Neutropenia | NA | NA | 3(7) | 2 (5) | 0(0) |
| Lymphocyte Count Decrease | 3(7) | 1(2) | 4(9) | 2 (5) | 0(0) |
| Neutrophile Count Decrease | 1(2) | 2(5) | 8(19) | 10(23) | 0(0) |
| Platelet Count Decrease | 4(9) | 1(2) | 3(7) | 3 (7) | 0(0) |
| White Blood Cell Decrease | 2(5) | 6(14) | 2(5) | 5 (12) | 0(0) |
Data are n (%). Data are for all exposed patients (n=41). Grade 1 or 2 adverse events occurring in at least 10% of patients and all grade 3 or worse adverse events are shown.
Two patients received the prohibited drug, ritonavir, a strong CYP3A4 inhibitor, in parallel with study treatment.15 Both patients developed febrile neutropenia, and one developed G3 pancreatitis prior to cycle 2. These 2 patients were removed from the study and final analysis, due to a violation of inclusion criteria, after receiving only one cycle of therapy. These patients were not included in the 41 patients enrolled on study (Figure 1). A third patient, changed to a non-ritonavir based regimen, only 3 days prior to treatment initiation, and developed prolonged G3 peripheral neuropathy. Subsequently, changes to the study to ensure safety were made including 1) ART and concomitant medications required approval prior to enrollment, 2) if there were 2 moderate CYP3A4 inhibitors at baseline, one had to be discontinued, (appendix p10), and 3) provider letters to infectious disease specialists to ensure ART was not changed during therapy without discussion. No ART-associated AE were noted after these changes were implemented.
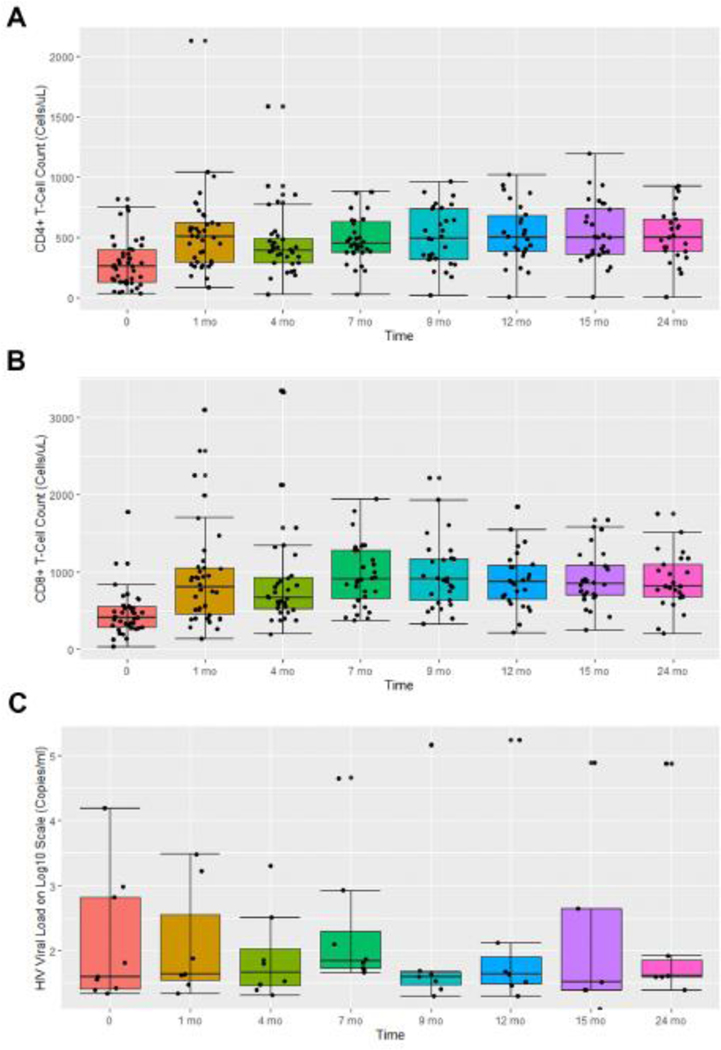
The median CD4+ and 8+ T-cell count at presentation was 258 cells/μl (Range 32–818) and 405 cell/μl (Range 26–1780), respectively (Table 1). Despite treatment with cytotoxic chemotherapy, 81% of the patients demonstrated elevations of both CD4+ and CD8+ T-cell counts by the end of treatment (Figure 3A). By cycle 2 day 1, 93% of the patients had CD4+ T-cell counts above baseline and the median CD4/8+ T-cell count increased from baseline of 258/405 cells/μl to 405/800 cells/μl, respectively (Figure 3A and B). The median CD4/8+ T-cell count elevation, remained stable for 18 months on post treatment evaluation. Fifteen patients of 41 (36%) had baseline CD4+ T-cell counts below 200 cells/μl (Range 32–179), consistent with the diagnosis of acquired immunodeficiency syndrome (AIDS). Twenty-eight days post treatment initiation, 13 of the 15 patients (86%) had CD4+ T-cell counts above 200 cells/μl (Range 254–530 cell/μl). All 13 patients continued to maintain CD4 and 8+ T-cell counts over 200 cells/μl up to 18 months post-therapy (appendix p13). Additionally, none of the patients had evidence of immune reconstitution syndrome. Of the 2 subjects whose CD4+ and CD8+ T-cell counts did not increase, one was non-compliant with ART as demonstrated by VL elevations during therapy, and the second had diarrhea and a G3 lymphopenia shortly prior to withdrawing from study.
Figure 3. Absolute number of CD4/8+ T-cells, and trends of patients with detectable HIV viral Loads at baseline, during therapy, and post-therapy evaluation.
Box plots assessing the pattern of (A) the absolute CD4+ T-cell counts (cells/μL), (B) CD8+ T-cell counts (cells/μL), and (C) detectable viral loads of the HIV-associated cHL patients enrolled in AMC085.
To better understand whether the increase in CD4 and 8+ T-cell counts was characteristic of ABVD-related chemotherapy, we analyzed, previously unpublished data of patients with HIV-cHL treated with ABVD from the Lymphovir and County Hospital AIDS Malignancy Project (CHAMP) cohorts (appendix p11,12).3,16 CD4 and 8+ T-cell count at baseline, during, and post chemotherapy were assessed. To avoid confounders related to poor baseline HIV control and ART related improvements, we only included patients with undetectable VL, implying compliance with ART, at baseline. Of the 55 patients treated with ABVD, the median CD4+ T-cell count decreased from 420 to 332 cells/μl (n=32) by end of therapy (Lymphovir), and the CD4 and 8+ T-cell count decreased from 262/442 to 202/350 cells/μl (n=23) during therapy (CHAMP), with a recovery back to baseline post chemotherapy for both cohorts (appendix p11,12).
In our study only 9 patients (22%) had baseline measurable HIV-1 VLs. The median VL was 52 copies/ml (range 22–15,700) with end of treatment VL range was 25–85 copies. In all patients with positive VL, these decreased during therapy, and remained undetectable in those with baseline undetectable viral loads (Figure 3C).
Discussion
AMC 085 is an open label phase II trial for the upfront treatment of stage II-IV cHL with BV-AVD, utilizing the same RP2D as determined in the non-HIV population, 1.2 mgs/kg.13 The trial demonstrated a 100% CR, 92% OS, and an 86% PFS with a median follow-up of 28 months for all patients. Results compare favorably with the BV-AVD arm of the ECHELON-1 study which had a CR rate of 73%.14 It is notable that the IPI scores of AMC 085 patients were higher than in the BV-AVD arm of ECHELON-1. Comparisons with the ECHELON-1 study and AMC-085, are made with the caveat that the former study was a large Phase 3 study. Rates of CR in advanced HIV-cHL, historically, are similar to those seen in the ECHELON-1 study, 75–86%5,17,18. Additionally, 10–15% of the non-HIV population treated with frontline therapy for advanced stage cHL had primary refractory disease, a finding not observed in the present study.11 This also confirms PET/CT scans can be utilized effectively in patients with HIV-associated Hodgkin lymphoma, as all subjects who completed therapy had negative scans. This data contradicts earlier concerns that false positive scans occur commonly in PLWH due to opportunistic infection or HIV itself.19 It is unclear if chemotherapy escalation based on a positive cycle 2 PET/CT may be indicated. AMC-085 demonstrated higher rates of survival compared to prospective trials of advanced stage HIV-cHL treated with ABVD, Stanford V, or risk-adapted approaches utilizing ABVD/BEACOOP.5,17,18 The 2-year PFS and OS in AMC085, was similar when compared to the BV-AVD arm of the ECHELON-1 study, 87% and 90% vs. 84% and 94% respectively.13,20
PN occurred 20% more often compared to the non-HIV population, however G3/4 PN in both the HIV and non-HIV population were just 10% vs. 5%, respectively, despite the known association of PN in the HIV+ population.21 The increase was mostly attributable to an elevation in G1/2 PN, which was managed with dose delays and reductions. G3/4 Neutropenia was 15% greater than in the non-HIV population. However, infectious complications (37 vs. 47%), hospitalizations (34 vs. 37%), deaths during therapy (1 vs. 2 %), febrile neutropenia (12 vs. 11%), were all similar, appendix p5. BV-AVD also removes bleomycin in a population with an elevated smoking prevalence, decreasing lung toxicity.22
Strong CYP3A4/p-glycoprotein inhibitors (e.g. ritonavir or cobisistat) can inhibit chemotherapy metabolism thus increasing toxicity. These drugs must be avoided.3,13,23,24 Early in the study, poor communication between the primary HIV provider and the oncologist and initiating treatment prior to the appropriate washout period of strong CYP3A4/p-glycoprotein inhibitors led to avoidable errors in ART administration which translated into SAE. Protocol amendments eliminated these problems. This correlates with PK studies demonstrating elevated levels of MMAE, when BV was administered in the presence of strong CYP 3A4 inhibitors and retrospective data demonstrating increased toxicity when vinblastine was co-administered with ritonavir.13,15,24,25
Surprisingly, CD4 and CD8+ T-cell counts increased in over 80% of patients while on cytotoxic chemotherapy and despite 44% of the subjects manifesting G3/4 neutropenia (Figure 3A and 3B). For patients enrolled with CD4+ T-cell counts below 200 cell/μl, 86% of the patients achieved CD4+ T-cell counts above 200 cells/μl by 1 month that were sustained over a year post therapy (appendix p13). The median CD8+ T-cell counts also doubled by 1 month, from 405 to 800 cells/μl (Figure 3B and appendix p13), not an effect noted with the administration of ART alone. In fact, historical controls of ABVD treated patients for 6 cycles demonstrated decreases in both median CD4 and 8+ T-cell counts (appendix p11,12) during therapy and a rebound back to normal post therapy, supporting the possibility of a BV specific mechanism, as opposed to a general treatment effect of HIV-cHL or secondary to ART. CD4+ T-cell decreases have been very well described during chemotherapy of HIV-associated lymphoma.3,26 Studies utilizing CHOP-R and DA-EPOCH-R demonstrate decreases in the median CD4+ and 8+ T-cell count and take 1 to 12 months post chemotherapy to recover, depending on concurrent or sequential ART, consistent with the CD4+ and 8+T-cell trends observed in the ABVD treated historical controls.3,16,25,26 In contrast, when used in the third to fourth line setting, single agent BV for relapse/refractory HIV-cHL was associated with decreases in CD4+ and CD8+ T-cells. T-regulatory cells (Tregs) have many functions, one of which is to suppress CD4+ and CD8+ T-cell proliferation.27 CD30 expression has been identified on CD4+ T-cells, including Tregs, and its expression on T-lymphocytes is augmented in the presence of viral infection, eg HIV.32–34 In the non-HIV setting, BV can selectively deplete CD30+ T-cells.27–29 Heiser et al, further demonstrated that CD30+ Tregs, both in vitro and in animal models, could be specifically depleted by BV, while inducing a concomitant CD8+ T-cell proliferation.30 Similarly, Tregs depletion, by BV in BV-AVD therapy, could provide explain the CD4+ and 8+ T-cell trends observed in AMC085 (Figure 3A and B and appendix p13). It should be noted however that it is unknown whether the laboratory finding of increased CD4+ and 8+ T-cell counts translates into improved immune function.
In summary, BV-AVD treatment demonstrated excellent outcomes in patients with stage II unfavorable to stage III/IV HIV-cHL with a CR rate of 100%, a 2-year OS and PFS of 92 and 86% respectively. In patients not on strong CYP3A4 inhibitors, the treatment was safe. The data suggest that the outcomes are as good or better than other regimens in HIV-cHL and at least comparable to those without HIV. Thus, these results add an important therapeutic option in the treatment of HIV-cHL. In addition, BV-AVD induced elevations in CD4+ and 8+ T-cells during treatment and in the post-treatment period for over 1 year compared with decreases in a historical controls of patients with HIV-cHL treated with ABVD.
Supplementary Material
Research in context.
Evidence before this study
HIV-associated Hodgkin remains the most common non-AIDS defining hematological malignancies in the HIV population. Prior studies in HIV-associated advanced stage Hodgkin lymphoma demonstrated an 80% 2-year OS with ABVD, the current standard of care. Risk adapted approaches utilizing BEACOPP resulted in unacceptable treatment-related mortality. The design of AMC 085 was based on the phase 1 study of BV-AVD which demonstrated a recommended phase 2 dose (RP2D) of brentuximab vedotin (BV) of 1.2 mgs/kg given in combination with doxorubicin, vinblastine, and decarbazine (AVD) with a manageable toxicity profile. Five year follow up of the phase 1 data demonstrated a complete remission rate and an event free survival superior to ABVD historical controls, which was the impetus for the BV-AVD vs. ABVD phase 3 ECHELON-1 study in advanced stage cHL. Recent data from the ECHELON-1 study demonstrated an overall survival benefit at 6 years over the standard of care, ABVD. Persons with HIV were excluded from all studies with BV-AVD. Searches in pubmed and clinical trials.gov demonstrated in 7/2012, 8/2013, 9/2017, 1/2022 no evidence of any study utilizing BV in the upfront setting in HIV-associated cHL.
Added value of this study
AMC-085 is the first study to evaluate BV-AVD as frontline therapy for HIV-associated cHL. We demonstrate, this regimen is safe in patients with HIV with similar 2 year outcomes as the ECHELON-1 study. This study also describes which particular ART should be avoided during therapy, and emphasized the necessity of myeloid growth factor, as the rate of neutropenia is more pronounced than in the non-HIV population. Unexpectedly, BV-AVD induces an increase in CD4 and CD8+ T-cells, in the setting of HIV and despite the concurrent use of lympho-toxic chemotherapy, AVD.
Implications of all the available evidence
We believe this study is practice changing, as HIV-cHL outcomes with BV-AVD compares favorably to earlier prospective studies in this population and demonstrates similar efficacy as the ECHELON-1 study, in the non-HIV population, at 2 years. Furthermore, the recommended phase 2 dose in AMC 085 was identical and safety was similar to the non-HIV population, adding more evidence that HIV as an exclusion criteria for prospective clinical trials should be re-examined. The unexpected increase in CD4 and 8+ T-cell counts noted during BV-AVD therapy compared to decreases in ABVD-treated cohorts, could also have implications in the care of HIV patients as a whole: few interventions aside from ART itself have ever demonstrated improved CD4+ T-cell counts in persons living with a HIV. The mechanism of this finding and its extended clinical implications of the observed CD4 and 8+ T cell increases deserves further exploration.
Acknowledgements
The study was coordinated by the AIDS Malignancy Consortium (AMC), the AMC Lymphoma Working Group, and the lymphoma study association (LYSA). All authors are supported in part by the NCI-sponsored AIDS Malignancy Consortium grant #UM1CA121947 and UM1CA181255 or the or the Lymphoma Study Association, LYSA (France). We would also like to acknowledge the French Agency for Research on AIDS and Viral Hepatitis (ANRS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Lymphoma Study Association.
Funding:
This work was supported in part by the National Institutes of Health (NIH), National Cancer Institute (NCI) awards UM1CA121947 and UM1CA181255 to the AMC and by LYSA.
AN has a MSK core grant NIH# P30 CA008748 and NIH grant #PO1 1568 G TA390. MAR is suppported by the JHU Core Grant #P30CA006973, contracts with Cullinan Apollo and RenovoRx, and is a founder and serves on the Board of Directors of Geminus Therapeutics, LLC. EGR is the co-chair of the NCCN panel of Cancer in Persons With HIV and Kaposi Sarcoma, an unpaid position. PCM MSK core grant P30CA008748. PGR, AN, EGR, PCM, MB, JYL, RM, DHH, JCR, RA, CD, MAR, AC, LR and EC are supported in part by NCI-sponsored AIDS Malignancy Consortium grant #UM1CA121947 and UM1CA181255. RFA Hopkins cancer center support 5P30CA006973. CB and NM are supported in part by LYSA, and DC and YT are supported in part by the ANRS. DC also has contracts with Jansen and speaker bureau funding from Giliad and Pfizer.
Footnotes
Data Sharing
Data collected for the study, including individual deidentified participant data and a data dictionary defining each field in the set, can be requested by email to the corresponding author. The protocol with the statistical plan is provided in the appendix.
Decleration of Interests.
All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olszewski, Adam J; Castillo, Jorge J. Outcomes of HIV-associated Hodgkin lymphoma in the era of antiretroviral therapy. AIDS. 2016;30:787–796. [DOI] [PubMed] [Google Scholar]
- 2.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV- infected population in the United States. Journal of the National Cancer Institute. 2011; 103:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besson C, Lancar R, Prevot S, Brice P, Meyohas MC, Marchou B, et al. High Risk Features Contrast With Favorable Outcomes in HIV-associated Hodgkin Lymphoma in the Modern cART Era, ANRS CO16 LYMPHOVIR Cohort. Clin Infect Dis. 2015. Nov 1; 61(9):1469–75. DOI: 10.1093/cid/civ627 [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. Aids. 2014; 28(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AM, Li P, et al. Chemotherapy consisting of doxorubicin, bleomycin, vinblastine, and dacarbazine with granulocyte-colony-stimulating factor in HIV-infected patients with newly diagnosed Hodgkin’s disease: a prospective, multi-institutional AIDS clinical trials group study (ACTG 149). J Acquir Immune Defic Syndr. 2000; 24(5): 444–50. [DOI] [PubMed] [Google Scholar]
- 6.Xicoy B, Ribera JM, Miralles P, Berenguer J, Rubio R, Mahillo B, et al. Results of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine and highly active antiretroviral therapy in advanced stage, human immunodeficiency virus related Hodgkin’s lymphoma. Haematologica 2007; 92:191–198. [DOI] [PubMed] [Google Scholar]
- 7.Montoto S, Shaw K, Okosun J, Gandhi S, Fields P, Wilson A, Shanyinde M et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012. Nov 20;30(33):4111–6. doi: 10.1200/JCO.2011.41.4193. Epub 2012 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hentrich M, Berger M, Wyen C, Siehl J, Rockstroh JK, Müller M, Fätkenheuer G et al. Stage-adapted Treatment of HIV-associated Hodgkin Lymphoma: Results of a Prospective Multicenter Study. J Clin Oncol. 2012;30:4117–23. doi: 10.1200/JCO.2012.41.8137. [DOI] [PubMed] [Google Scholar]
- 9.Merli F, Luminari S, Gobbi PG et al. Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: a study by Fondazione Italiana Linfomi. J Clin Oncol. 2016; 34(11): 1175–1181. [DOI] [PubMed] [Google Scholar]
- 10.Adcetris (brentuximab vedotin). Both- ell, WA: Seattle Genetics, 2016. (package insert) (http://www.seattlegenetics.com/application/files/2515/1059/6728/ADCETRIS_USPI_USP-BVP-2015-01625pdf.pdf).
- 11.Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. NEJM.2018; 378:1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, Radford J, Connors JM, Długosz-Danecka M, Kim WS, Gallamini A, et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N Engl J Med. 2022. Jul 28;387(4):310–320. doi: 10.1056/NEJMoa2206125. Epub 2022 Jul 13. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein PG, Moore PC, Rudek MA, Henry DH, Ramos JC, Ratner L, Reid E et al. Brentuximab vedotin with AVD shows safety, in the absence of strong CYP3A4 inhibitors, in newly diagnosed HIV-associated Hodgkin lymphoma. AIDS. 2018. Mar 13;32(5):605–611. doi: 10.1097/QAD.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 15.Han TH, Gopal AK, Ramchandren R, Goy A, Chen R, Matous JV, Cooper M, Grove LE, Alley SC, Lynch CM, O’Connor OA. CYP3A-mediated drug-drug interaction potential and excretion of brentuximab vedotin, an antibody-drug conjugate, in patients with CD30-positive hematologic malignancies. J Clin Pharmacol. 2013. Aug;53(8):866–77. doi: 10.1002/jcph.116. Epub 2013 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarco CE, Lu P, Peace DF, Singh S, Angelov DF, Benante KA, Ahmed AT, Rubinstein PG. Characteristics, Social Factors, and Trends in HIV and AIDS-Related Lymphoma: A 23-Year Analysis Since the Implementation of c ART, a County Hospital AIDS Malignancy Project (CHAMP) Study. Blood (2018) 132 (Supplement 1): 2304. [Google Scholar]
- 17.Hentrich M, Berger M, Wyen C, Siehl J, Rockstroh JK, Müller M, Fätkenheuer G et al. Stage-adapted Treatment of HIV-associated Hodgkin Lymphoma: Results of a Prospective Multicenter Study. J Clin Oncol. 2012;30:4117–23. doi: 10.1200/JCO.2012.41.8137. [DOI] [PubMed] [Google Scholar]
- 18.Spina M, Gabarre J, Rossi G, Fasan M, Schiantarelli C, Nigra E, Mena M, Antinori A, Ammassari A, Talamini R, Vaccher E, di Gennaro G, Tirelli U. Stanford V Regimen and Concomitant HAART in 59 Patients With Hodgkin Disease and HIV Infection. Blood. 2002. Sep 15;100(6):1984–8. doi: 10.1182/blood-2002-03-0989 [DOI] [PubMed] [Google Scholar]
- 19.Warwick JM, Sathekge MM. PET/CT scanning with a high HIV/AIDS prevalence. Transfus Apher Sci. 2011. Apr;44(2):167–72. doi: 10.1016/j.transci.2011.01.014. Epub 2011 Feb 22. [DOI] [PubMed] [Google Scholar]
- 20.Connors JM, Younes A, Gallamini A, Ansell SM, Kim WS, Advani RH, Bartlett NL et al. Brentuximab Vedotin Plus Chemotherapy in Patients with Advanced-Stage Classical Hodgkin Lymphoma (cHL): Evaluation of Modified Progression-Free Survival (mPFS) and Traditional PFS in the Phase 3 ECHELON-1 Study. Blood (2018) 132 (Supplement 1): 2904. [Google Scholar]
- 21.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011. Apr 24;25(7):919–28. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mdodo Rennatus, Frazier Emma L., Dube Shanta R., et al. Cigarette Smoking Prevalence Among Adults With HIV Compared With the General Adult Population in the United States: Cross-sectional Surveys. Ann Intern Med.2015;162:335–344.. doi: 10.7326/M14-0954 [DOI] [PubMed] [Google Scholar]
- 23.Powles T, Imami N, Nelson M, Gazzard BG, Bower M. Effects of combination chemotherapy and highly active antiretroviral therapy on immune parameters in HIV-1 associated lymphoma. AIDS. 2002;16:531–536. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein PG, Braik T, Jain S et al. Ritonavir Based Highly Active Antiretroviral Therapy (HAART) Correlates with Early Neurotoxicity When Combined with ABVD Treated HIV Associated Hodgkin Lymphoma but Not Non-Hodgkin Lymphoma. A Retrospective Study. American Society of Hematology 2010. Abstract # 2807.
- 25.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011; 12(9):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan CRC, Barta SK, Lee J, Rudek MA, Sparano JA, Noy A. Combination antiretroviral therapy accelerates immune recovery in patients with HIV-related lymphoma treated with EPOCH: a comparison within one prospective trial AMC034. Leuk Lymphoma. 2018. Aug;59(8):1851–1860. doi: 10.1080/10428194.2017.1403597. Epub 2017 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. Epub 2012 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prator CA, Thanh C, Kumar S, Pan T, Peluso MJ, Bosch R, Jones N, Milush JM, Bakkour S, Stone M, Busch MP, Deeks SG, Hunt PW, Henrich TJ. Circulating CD30+CD4+ T Cells Increase Before Human Immunodeficiency Virus Rebound After Analytical Antiretroviral Treatment Interruption. J Infect Dis. 2020. Mar 16;221(7):1146–1155. doi: 10.1093/infdis/jiz572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogan LE, Vasquez J, Hobbs KS, Hanhauser E, Aguilar-Rodriguez B, Hussien R, et al. (2018) Increased HIV-1 transcriptional activity and infectious burden in peripheral blood and gut-associated CD4+ T cells expressing CD30. PLoS Pathog 14(2): e1006856. 10.1371/journal.ppat.1006856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiser RA, Grogan BM, Manlove LS, Gardai SJ. Abstract 1789: CD30+T regulatory cells, but not CD30+CD8 T cells, are impaired following brentuximab vedotin treatment in vitro and in vivo. Cancer Res. July 1 2018. (78) (13 Supplement) 1789; DOI: 10.1158/1538-7445.AM2018-1789 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.