Abstract
Purpose
This study aims to investigate the relationship between thyroid and type 1 diabetic nephropathy (T1DN) in euthyroid populations, focusing on thyroid hormone sensitivity.
Methods
A cross-sectional study was conducted between January 2016 and December 2021, including 357 euthyroid patients with type 1 diabetes mellitus (T1DM). Parameters representing thyroid hormone sensitivity were assessed, including the thyroid feedback quantile-based index (TFQI), parameter thyroid feedback quantile index (PTFQI), thyroid stimulating hormone index (TSHI), thyrotropin thyroxine resistance index (TT4RI), and free triiodothyronine/free thyroxine (FT3/FT4). Logistic regression and restricted cubic spline regression were performed to detect the association between thyroid hormone sensitivity and the risk of T1DN.
Results
The study found a negative correlation between the risk of T1DN and FT3/FT4 in euthyroid T1DM patients (OR 0.71, 95% CI 0.51–0.97, P <0.01). PTFQI (P<0.05), TSHI (P<0.05), and TT4RI (P<0.01) showed an M-shaped nonlinear relationship with the risk of T1DN. Elevated risk of T1DN was associated with PTFQI, TSHI, and TT4RI values outside the range of zero, 2.3–3.88, and 27.56–32.19, respectively.
Conclusion
This study confirms the relationship between impaired thyroid hormone sensitivity and the risk of T1DN in euthyroid patients. It emphasizes the importance of evaluating thyroid hormone sensitivity in T1DM patients, even when their thyroid function appears normal, to promptly prevent the occurrence of T1DN.
Keywords: euthyroid, sensitivity to thyroid hormones, type 1 diabetic nephropathy
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized by severe paucity of endogenous insulin and subsequent hyperglycemia.1 The global incidence of T1DM has been increasing, with approximately 8.4 million individuals worldwide affected in 2021.2 It is predicted that the number of cases will rise to 13.5–17.4 million by 2040.2 This disease imposes a significant social burden and economic pressure. Diabetic nephropathy (DN) is one of the most common and severe chronic complications of T1DM, often leading to end-stage kidney disease (ESKD).3,4 Despite well-controlled blood sugar and blood pressure levels, and advancements in the pharmaceutical field to prevent diabetes microvascular disease, the prevalence of type 1 diabetic nephropathy (T1DN) remains high, affecting around 50% of individuals and potentially exceeding 70%.5–7
In recent years, research has indicated a potential association between thyroid stimulating hormone (TSH) or free triiodothyronine (FT3) with the risk of T1DN.8,9 Yang et al suggested that FT3, free thyroxine (FT4), and TSH could simply reflect the thyroid function, while a new perspective on thyroid status was provided by evaluating sensitivity to thyroid hormone.10 Sensitivity to thyroid hormone can systematized into central thyroid hormone sensitivity, reflecting the negative feedback regulation of the central nervous system, and peripheral thyroid hormone sensitivity, reflecting the metabolic effects of hormones.11 The thyrotropin thyroxine resistance index (TT4RI) and the thyroid stimulating hormone index (TSHI) were identified as measures of central thyroid hormone sensitivity.12,13 Similarly, Laclaustra et al proposed the thyroid feedback quantile-based index (TFQI) and parameter thyroid feedback quantile index (PTFQI) to accurately assess central sensitivity to thyroid hormones, and also confirm the association of TFQI with diabetes and diabetes mortality.11 The above indicators of central sensitivity to thyroid hormones are calculated through FT4 and TSH. The ratio of FT3 and FT4 can estimate the conversion efficiency of FT4 to FT3, which indirectly represents the peripheral sensitivity of thyroid hormones.14 Previous studies have reported that impaired sensitivity to thyroid hormones is linked to metabolic disorders, such as prediabetes, obesity, and metabolic syndrome.11,15,16
However, the relationship between sensitivity to thyroid hormones and the risk of T1DN remains uncertain. Therefore, the purpose of this study was to investigate the association between indicators of thyroid hormone sensitivity and the risk of T1DN in patients with T1DM and normal thyroid function.
Method
Study Population
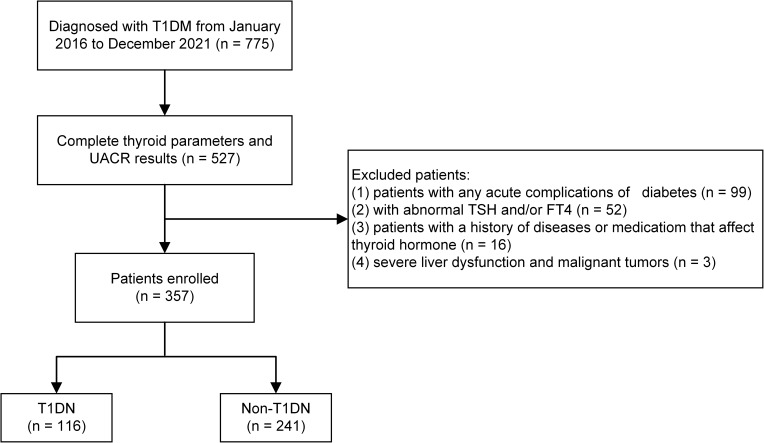
A total of 775 patients diagnosed with T1DM at the Department of Endocrinology, Gansu Provincial Hospital, Lanzhou, China from January 2016 to December 2021 were initially enrolled for this study. Patients diagnosed with T1DM17 were included and the exclusion criteria were as follows: (1) with any acute complications of diabetes; (2) with abnormal thyroid function (TSH, FT4, and FT3 exceeding the range of 0.35–4.94 mIU/L, 9.01–19.05 pmol/L, and 2.41–6.01 pmol/L, respectively); (3) with a history of diseases or medication that affect thyroid hormone levels; (4) kidney damage due to non-diabetes; (5) pregnant and breastfeeding women; (6) severe liver dysfunction and malignant tumors; and (7) lack of measurements of TSH, FT3, FT4 or urinary albumin to creatinine ratio (UACR). Ultimately, 357 subjects were included in the study (Figure 1). The current study was approved by the Medical Ethics Committee of Gansu Provincial Hospital and compliance with the Declaration of Helsinki. Given the retrospective nature of this study, the requirement for written informed consent was waived by the Medical Ethics Committee of Gansu Provincial Hospital (No. 2022–473).
Figure 1.
Flowchart of the inclusion and exclusion of participants.
Data Collection and Definitions
Demographic data, clinical data, and laboratory examination data of patients were collected. Demographic and clinical data included age, gender, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and duration of T1DM. Blood pressure (BP) was measured after at least five minutes of rest and averaged twice BP reading measured at an interval of two minutes. Hypertension was defined as SBP ≥140 mmHg, DBP ≥90 mmHg, or the use of antihypertensive drugs. Body mass index (BMI) was calculated by dividing body weight in kilograms by the square of height in meters.
Venous blood samples were collected in the morning after an 8 to 12-hour fast. An automatic biochemical analyzer (Abbott Laboratories, USA) was utilized for biochemical parameters measurement, including fasting C-peptide (FC-P), fasting blood glucose (FBG), 2-hour postprandial blood glucose (2hPBG), glycated hemoglobin (HbAlc), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), total protein (TP), serum albumin (ALB), serum creatinine (SCr) and uric acid (UA). Serum levels of FT3, FT4, TSH, and thyroid peroxidase antibody (TPOAb) were determined on the IMMULITE 2000 system (Abbott Laboratories, USA) using Chemiluminescent Immunoassay detection methods.
Euthyroid was defined as having FT4 (9.01–19.05 pmol/L), FT3 (2.41–6.01 pmol/L), and TSH (0.35–4.94 mIU/L) within normal ranges, and without a known history of thyroid disease. DN was diagnosed with the UACR ≥30mg/g for at least 3 months.18
Assessment of Thyroid Hormone Sensitivity Indices
The cumulative distribution function (cdf) was used to assess TFQI. TFQ1=(cdf)FT4-(1-cdfTSH).11 It ranges from −1 to 1, with negative values indicating an increase in central sensitivity to thyroid hormone, positive values indicating a decrease, and 0 values indicating normal sensitivity. PTFQI was calculated using the standard normal cumulative distribution function with FT4 and TSH. PTFQI=Ф((FT4-µFT4)/σFT4)-(1-Ф((lnTSH-µlnTSH)/σlnTSH)).11 In the population, μFT4 = 13.99, σFT4 = 2.76, μln TSH = 0.66, and σln TSH = 0.65. PTFQI can be easily calculated using the Excel or LibreOffice spreadsheet formula =NORM.DIST(fT4_cell, µfT4, σfT4, TRUE)+NORM.DIST(LN(TSH_cell)µlnTSH, σlnTSH, TRUE)-1. It is an approximation created to improve the applicability of the study and has the same range and interpretation as TFQI.
TSHI was calculated as lnTSH(mIU/L)+0.1345xFT4(pmol/L), and TT4RI was FT4(pmol/L)xTSH(mIU/L).12,13 Elevated TSHI or TT4RI levels indicate reduced central sensitivity to thyroid hormones.
The ratio of FT3 to FT4 was calculated to evaluate peripheral thyroid hormone sensitivity, and it showed a positive correlation with peripheral thyroid hormone sensitivity.
Statistical Analysis
The normality of variables was evaluated using the Shapiro–Wilk test. Continuous variables with a normal distribution were described as mean ± standard deviations (SDs), and Student’s t-test was used to compare the two groups. Otherwise, they were represented by median (interquartile ranges, IQRs) and the Mann–Whitney U-test was used. Data of categorical variables were presented as numbers (%) and the Chi-square test was applied to compare groups.
Logistic regression was performed to analyze the relationship between thyroid hormone sensitivity indicators and the risk of T1DN in three models. The models included an unadjusted model, a model adjusted for age and gender, and a model adjusted for age, gender, BMI, Duration, SBP, DBP, HR, FC-P, FBG, 2hPBG, HbA1c, TPOAb positive, TC, TG, LDL-C, ALT, AST, TBIL, DBIL, TP, ALB, SCr and UA. The results were expressed as odds ratio (OR) and 95% confidence interval (CI). A restricted cubic spline (RCS) model was used to analyze the dose-response relationship between thyroid hormone sensitivity indicators and the risk of T1DN. The number of nodes was set at 5, and the nodes were divided equally by quantiles. P <0.05 (two-sided) was considered statistically significant. All analyses were conducted using SPSS 26.0 and R (version 4.2.1) statistical software.
Results
Characteristics of the Study Population
A total of 357 individuals took part in this cross-sectional survey. Among them, 116 patients (32.5%) were diagnosed with T1DN. The average age of these patients was 29 years, and 50.9% of them were male. The findings revealed that T1DN patients tended to be older and had a longer duration of diabetes. Additionally, the T1DN group exhibited elevated levels of DBP, FBG, and SCr, while experiencing a significant decrease in FC-P and TBIL levels, as well as FT3/FT4 values (P <0.05). The characteristics of the participants are summarized in Table 1.
Table 1.
Clinical Characteristics of Participants in the Study
| Overall (n=357) | Non-T1DN (n=241) | T1DN (n=116) | P value | |
|---|---|---|---|---|
| Men, n (%) | 191 (53.50%) | 132 (54.77%) | 59 (50.86%) | 0.48 |
| Age, year | 25 (16, 36) | 22 (15, 34) | 29 (20, 39) | <0.001 |
| Duration, year | 4 (1, 9) | 3 (1, 7) | 6.5 (2, 13.75) | <0.001 |
| Han nationality, n (%) | 327 (91.60%) | 225 (93.36%) | 102 (87.93%) | 0.18 |
| BMI, Kg/m2 | 20.27±3.89 | 20.19±4.27 | 20.44±2.94 | 0.57 |
| SBP, mmHg | 114 (104, 124) | 111 (101, 121) | 117 (106.25, 132) | 0.31 |
| DBP, mmHg | 74.66±12.97 | 73.51±12.45 | 77.05±13.75 | 0.02 |
| HR, bmp | 90.95±16.81 | 90.77±16.70 | 91.33±17.21 | 0.77 |
| FC-P, ng/mL | 0.29 (0.03, 0.53) | 0.29 (0.03, 0.55) | 0.23 (0.04, 0.48) | 0.03 |
| FBG, mmol/L | 12.40 (8.32, 15.80) | 11.95 (8.20, 15.04) | 13.00 (9.64, 16.97) | 0.04 |
| 2hPBG, mmol/L | 14.92 (9.75, 18.68) | 14.92 (10.02, 19.03) | 14.23 (8.83, 18.05) | 0.35 |
| HbA1c, % | 10.79±2.93 | 10.69±3.02 | 11.01±2.74 | 0.33 |
| TC, mmol/L | 4.40±1.08 | 4.07±1.04 | 4.16±1.28 | 0.46 |
| TG, mmol/L | 1.21 (0.83, 1.74) | 1.19 (0.83, 1.74) | 1.22 (0.83, 1.80) | 0.58 |
| LDL-C, mmol/L | 2.43 (1.88, 2.94) | 2.34 (1.87, 2.89) | 2.55 (1.87, 3.14) | 0.76 |
| HDL-C, mmol/L | 1.22 (1.00, 1.51) | 1.23 (1, 1.52) | 1.19 (0.99, 1.47) | 0.78 |
| ALT, U/L | 17 (11.7, 27.64) | 16 (11.7, 27.64) | 18 (11.25, 33) | 0.67 |
| AST, U/L | 20 (15, 27.09) | 20 (15, 27) | 21 (16, 27.09) | 0.44 |
| TBIL, μmol/L | 3.8 (2.6, 5.0) | 14.22 (10.25, 18.25) | 11.1 (8.2, 14.28) | <0.001 |
| DBIL, μmol/L | 13.4 (9.6, 16.8) | 3.9 (2.6, 5.2) | 3.65 (2.63, 4.90) | 0.70 |
| TP, g/L | 69.38 (63.40, 73.67) | 69.38 (63.5, 73.3) | 68.75 (63.1, 74.33) | 0.42 |
| ALB, g/L | 42.05 (38.10, 47.70) | 42.4 (38.6, 45.7) | 41.4 (37.13, 45.60) | 0.56 |
| SCr, μmol/L | 54.6 (41.5, 66) | 51 (37, 63.4) | 59.25 (48.33, 74.68) | <0.001 |
| UA, μmol/L | 277.5 (216.50, 351.31) | 270 (206.5, 346) | 296.16 (230, 361.75) | 0.34 |
| FT4, pmol/L | 13.99±2.76 | 13.81±2.76 | 14.36±2.73 | 0.08 |
| FT3, pmol/L | 4.3±1.16 | 4.35±1.20 | 4.19±1.08 | 0.22 |
| TSH, mIU/L | 2.03 (1.38, 3.06) | 2.04 (1.41, 2.93) | 1.98 (1.27, 3.27) | 0.77 |
| TPOAb positive, (n, %) | 45 (12.6%) | 36 (14.94%) | 9 (7.76%) | 0.06 |
| TFQI | −0.05±0.43 | −0.01±0.41 | 0.19 (−0.36, 0.44) | 0.35 |
| PTFQI | −0.05±0.41 | −0.003±0.40 | 0.14 (−0.34, 0.40) | 0.37 |
| TSHI | 2.6 (2.09, 3.1) | 2.52±0.77 | 2.58±0.8 | 0.54 |
| TT4RI | 27.56 (18.61, 44.68) | 27.44 (19.19, 43.31) | 29.16 (16.53, 49.82) | 0.41 |
| FT3/FT4 | 0.31±0.09 | 0.32±0.09 | 0.29±0.07 | 0.008 |
Notes: Data are expressed as the mean ± standard deviations or medians (interquartile ranges) or numbers (%).
Abbreviations: T1DN, type 1 diabetic nephropathy; Non-T1DN, non-type 1 diabetic nephropathy; BMI, Body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; FC-P, fasting c-peptide; FBG, fasting blood glucose; 2hPBG, 2-hour postprandial blood glucose; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, glutamic pyruvic transaminase; AST, glutamic oxaloacetic transaminase; TBIL, total bilirubin; DBIL, direct bilirubin; TP, total protein; ALB, serum albumin; SCr, serum creatinine; UA, uric acid; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TPOAb, thyroid peroxidase antibody; TFQI, thyroid feedback quantile-based index; PTFQI, parameter thyroid feedback quantile index; TSHI, thyroid stimulating hormone index; TT4RI, thyrotropin thyroxine resistance index; FT3/FT4, free triiodothyronine/free thyroxine.
Association of Sensitivity to Thyroid Hormones with the Risk of T1DN
The FT3/FT4 value was found to be negatively associated with the risk of T1DN patients with normal thyroid function. For each standard deviation in the FT3/FT4 ratio, the risk of T1DN was reduced by 0.29 times (95% CI 0.51–0.97, P<0.01). However, there was no statistical significance between the risk of T1DN and TFQI, PTFQI, TSHI, or TT4RI In the three logistic models (P > 0.05). Please refer to Table 2 for detailed results.
Table 2.
Association Between Indicators of Sensitivity to Thyroid Hormone and the Risk of T1DN
| Variables | T1DN | |||||
|---|---|---|---|---|---|---|
| OR (95% CI)a +1SD | P value | OR (95% CI)b+1SD | P value | OR (95% CI)c+1SD | P value | |
| TFQI | 1.11 (0.89–1.39) | 0.35 | 1.13 (0.89–1.43) | 0.3 | 1.09 (0.82–1.45) | 0.54 |
| PTFQI | 1.11 (0.88–1.38) | 0.37 | 1.13 (0.89–1.42) | 0.32 | 1.08 (0.82–1.43) | 0.57 |
| TSHI | 1.07 (0.86–1.34) | 0.54 | 1.06 (0.84–1.35) | 0.62 | 1.03 (0.78–1.36) | 0.83 |
| TT4RI | 1.10 (0.88–1.37) | 0.41 | 1.08 (0.86–1.36) | 0.48 | 1.07 (0.82–1.39) | 0.63 |
| FT3/FT4 | 0.71 (0.55–0.91) | 0.008 | 0.70 (0.55–0.92) | 0.008 | 0.71 (0.51–0.97) | 0.02 |
Notes: aUnadjusted; bAdjusted for age, gender; cAdjusted for age, gender, BMI, Duration, SBP, DBP, HR, FC-P, FBG, 2hPBG, HbA1c, TC, TG, LDL-C, ALT, AST, TBIL, DBIL, TP, ALB, SCr, UA and TPOAb.
Abbreviations: OR, Odds ratios; CI, Confidence interval; SD, standard deviation; T1DN, type 1 diabetic nephropathy; TFQI, thyroid feedback quantile-based index; PTFQI, parameter thyroid feedback quantile index; TSHI, thyroid stimulating hormone index; TT4RI, thyrotropin thyroxine resistance index; FT3/FT4, free triiodothyronine/free thyroxine.
Nonlinear Relationship Between Sensitivity to Thyroid Hormones and the Risk of T1DN
An M-shaped nonlinear dose-response relationship was observed between PTFQI, TSHI, or TT4RI and the risk of T1DN in euthyroid patients using RCS regression analysis. The lowest risk of T1DN was associated with a PTFQI value of 0 (OR = 1). However, the risk of T1DN increased for other values of PTFQI (P <0.05). Furthermore, there was a significant correlation between TSHI values outside the range of 2.30–2.88 and an increased risk of T1DN (P < 0.05). Similarly, TT4RI values outside the range of 27.56–32.19 were positively associated with the risk of T1DN (P < 0.01). (Figure 2)
Figure 2.
Nonlinear relationship between PTFQI (A), TSHI (B), or TT4RI (C) and the risk of T1DN in euthyroid patients with T1DM. OR were adjusted for age, gender, BMI, Duration, SBP, DBP, HR, FC-P, FBG, 2hPBG, HbA1c, TC, TG, LDLC, ALT, AST, TBIL, DBIL, TP, ALB, SCr, UA, and TPOAb.
Discussion
In this cross-sectional investigation, we found that the risk of T1DN in T1DM patients with normal thyroid function was associated with thyroid hormone sensitivity. The ratio of FT3 to FT4, which indicates peripheral thyroid hormone sensitivity, showed a significant inverse relationship with the risk of T1DN. Additionally, an M-shaped nonlinear relationship was found between central thyroid hormone sensitivity (PTFQI, TSHI, and TT4RI) and the risk of T1DN. These innovative findings could serve as a theoretical guide for the early detection and treatment of T1DN in T1DM patients with normal thyroid function.
Previous research has focused on the relationship between FT3, FT4, or TSH levels and T1DM complications. For instance, a study demonstrated that TSH levels (0.4–2.5 mU/L) were associated with a lower risk of diabetic retinopathy and renal failure in individuals with T1DM.8 Another study by Bogusz Falkowski found that T1DM patients with normal thyroid function had a lower incidence of microangiopathy when their FT3 concentrations were higher.9 Intriguingly, our results indicate that in euthyroid T1DM patients, neither FT3, FT4, nor TSH levels were related to an increased risk of T1DN. This suggests that TSH, FT3, and FT4 might not adequately reflect the connection between thyroid status and T1DN.
Recently, there has been a growing focus on studying the correlation between thyroid hormone sensitivity and metabolic diseases.19–21 A study indicated that higher levels of TSHI and TT4RI were associated with kidney disorders in patients with type 2 diabetes mellitus (T2DM).22 And Zhao et al showed a decrease in the FT3/FT4 is an independent predictor of diabetic kidney disease occurrence in patients with T2DM.23 In euthyroid individuals, increased levels of PTFQI, TSHI, TT4RI, and decreased levels of FT3/FT4 were found to be linked to kidney diseases.10 However, the relationship was not found in another study.24 Additionally, a recent study discovered a non-linear association between TFQI and the incidence of diabetic retinopathy.25 However, another cross-sectional investigation revealed that TFQI, TSHI, TT4RI, and FT3/FT4 were all connected to the risk of diabetic retinopathy.26 In patients with T2DM, the prevalence of diabetic peripheral neuropathy was negatively correlated with FT3/FT4.27 It is worth affirming that there is a definite relationship between thyroid hormone sensitivity and microvascular disease in diabetes. Therefore, this innovative research aims to explore the relationship between thyroid hormone sensitivity and the risk of T1DN in euthyroid T1DM patients, contributing to our understanding of thyroid hormone sensitivity and microvascular disease in diabetes.
The mechanism of the association between sensitivity to thyroid hormones and T1DN with normal thyroid function is currently unclear. Still, interpretations might be provided by some potential mechanisms. Recent research has confirmed that thyroid hormones can regulate endothelial function and podocyte redifferentiation, both of which play crucial roles in the pathophysiology of diabetic nephropathy.28–30 Thyroid hormones can downregulate the expression of angiotensin II type 1 receptor (AT1R) and relax blood vessels, thereby regulating endothelial function.29 Additionally, they can induce podocyte redifferentiation, decrease hypertrophy, and enhance renal structure.30 Another important finding is that 3,5-triiodothyronine has been shown to protect the kidney by regulating Sirtuin 1 (SIRT1) in diabetic rats and rat mesangial cells exposed to high glucose.31 Moreover, intrarenal oxidative stress significantly influences the initiation and progression of diabetic kidney disease.32 Deiodination of FT4 results in the formation of the more active FT3, and Type II deiodinase (DIO2) plays a key role in this conversion.33 The intracellular activation of thyroid hormone by DIO2 reduces the cell’s reliance on aerobic glycolysis, lowering mitochondrial respiration and reactive oxygen species generation and thereby limiting oxidative stress to the best extent possible.34 The FT3/FT4 ratio represents DIO2 activity,35 and a decrease in this ratio reflects a reduction in DIO2 activity, leading to increased oxidative stress and inflammatory response, thereby promoting the occurrence and development of DN.
The strength of this study was to assess the relationship between sensitivity to thyroid hormones and the risk of T1DN in euthyroid T1DM populations, as well as investigate their dose-response relationship. However, there are some limitations to consider. Firstly, as a cross-sectional study, it cannot establish a causal relationship between impaired sensitivity to thyroid hormones and the risk of T1DN in individuals with normal thyroid function. Even though we controlled for various covariates, the possibility of reverse causation and pharmaceutical influence cannot be ruled out. Nonetheless, the research supports the significant hypothesis that adding the evaluation of thyroid hormone sensitivity levels to the examination of thyroid hormone levels may be helpful in assessing the risk of T1DN. Secondly, due to the retrospective nature of the study, certain relevant laboratory examination data were not collected. Thirdly, the limited sample size may have resulted in imprecise results, which restricts the generalizability of the findings. Finally, since the data was obtained from Gansu province, it may not be representative of the entire population. Therefore, further prospective research involving multiple centers and larger sample sizes is necessary to determine and validate this relationship.
Conclusions
In general, our study found that the risk of T1DN was associated with impaired central and peripheral sensitivity to thyroid hormones in euthyroid T1DM individuals. The indicators of sensitivity to thyroid hormones provide a more systematic and comprehensive method for evaluating thyroid status compared to TSH, FT4, or FT3. Therefore, they may be considered as a complementary approach to evaluating the development of T1DN in clinical practices. In the future, large prospective studies are indispensable to confirm these findings.
Acknowledgment
We appreciate the efforts of the staff and participants for their contributions to this study.
Funding Statement
This work was supported by the Major Science and Technology Projects in Gansu Province (No. 22ZD6FA033).
Data Sharing Statement
All the additional data are available from the corresponding author upon reasonable request.
Ethics Declarations
The study was approved by the Ethics Committee of Gansu Provincial Hospital and compliance with the Declaration of Helsinki. Given the retrospective nature of this study, the requirement for written informed consent was waived by the Ethics Committee of Gansu Provincial Hospital. To preserve the patient’s privacy, we de-identified and anonymized patient records/information before analysis. (No. 2022-473)
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. doi: 10.1016/s0140-6736(18)31320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory GA, Robinson TIG, Linklater SE, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741–760. doi: 10.1016/s2213-8587(22)00218-2 [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes/Metab Res Rev. 2017;33(2):567. [DOI] [PubMed] [Google Scholar]
- 4.Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obesity Metab. 2020;22(Suppl 1):3–15. doi: 10.1111/dom.14007 [DOI] [PubMed] [Google Scholar]
- 5.Costacou T, Orchard TJ. Cumulative Kidney Complication Risk by 50 Years of Type 1 Diabetes: the Effects of Sex, Age, and Calendar Year at Onset. Diabetes Care. 2018;41(3):426–433. doi: 10.2337/dc17-1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sever B, Altıntop MD, Demir Y, Akalın Çiftçi G, Beydemir Ş, Özdemir A. Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds. Bioorg Chem. 2020;102:104110. doi: 10.1016/j.bioorg.2020.104110 [DOI] [PubMed] [Google Scholar]
- 7.Demir Y, Ceylan H, Türkeş C, Beydemir Ş. Molecular docking and inhibition studies of vulpinic, carnosic and usnic acids on polyol pathway enzymes. J Biomol Struct Dyn. 2022;40(22):12008–12021. doi: 10.1080/07391102.2021.1967195 [DOI] [PubMed] [Google Scholar]
- 8.Rodacki M, Zajdenverg L, Dantas JR, et al. Should thyroid-stimulating hormone goals be reviewed in patients with type 1 diabetes mellitus? Results from the Brazilian Type 1 Diabetes Study Group. Diabetic Med. 2014;31(12):1665–1672. doi: 10.1111/dme.12530 [DOI] [PubMed] [Google Scholar]
- 9.Falkowski B, Rogowicz-Frontczak A, Grzelka A, et al. Higher free triiodothyronine concentration is associated with lower prevalence of microangiopathic complications and better metabolic control in adult euthyroid people with type 1 diabetes. Endocrine. 2018;60(3):458–465. doi: 10.1007/s12020-018-1582-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S, Lai S, Wang Z, Liu A, Wang W, Guan H. Thyroid Feedback Quantile-based Index correlates strongly to renal function in euthyroid individuals. Annals of Medicine. 2021;53(1):1945–1955. doi: 10.1080/07853890.2021.1993324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired Sensitivity to Thyroid Hormones Is Associated With Diabetes and Metabolic Syndrome. Diabetes Care. 2019;42(2):303–310. doi: 10.2337/dc18-1410 [DOI] [PubMed] [Google Scholar]
- 12.Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J Clin Endocrinol Metab. 1997;82(5):1608–1614. doi: 10.1210/jcem.82.5.3945 [DOI] [PubMed] [Google Scholar]
- 13.Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin. Endocrinol. 2009;71(4):529–534. doi: 10.1111/j.1365-2265.2009.03534.x [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Park SE, Jung SW, et al. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin. Endocrinol. 2017;87(1):87–96. doi: 10.1111/cen.13345 [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Wang Z, Fu J, Guan H, Lyu Z, Wang W. Sensitivity to Thyroid Hormones and Risk of Prediabetes: a Cross-Sectional Study. Front Endocrinol. 2021;12:657114. doi: 10.3389/fendo.2021.657114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corica D, Licenziati MR, Calcaterra V, et al. Central and peripheral sensitivity to thyroid hormones and glucose metabolism in prepubertal children with obesity: pilot multicenter evaluation. Endocrine. 2023;80(2):308–311. doi: 10.1007/s12020-022-03276-5 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–s33. doi: 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 18.de Boer IH, Khunti K, Sadusky T, et al. Diabetes Management in Chronic Kidney Disease: a Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–3090. doi: 10.2337/dci22-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Chen Y, Ye H, et al. Correlation between thyroid function, sensitivity to thyroid hormones and metabolic dysfunction-associated fatty liver disease in euthyroid subjects with newly diagnosed type 2 diabetes. Endocrine. 2023;80(2):366–379. doi: 10.1007/s12020-022-03279-2 [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Li Z, Yang R, et al. Impaired Sensitivity to Thyroid Hormones Is Associated With Elevated Blood Glucose in Coronary Heart Disease. Front Endocrinol. 2022;13:895843. doi: 10.3389/fendo.2022.895843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Jiang Y, Li P, et al. Association of impaired sensitivity to thyroid hormones with hyperuricemia through obesity in the euthyroid population. J Transl Med. 2023;21(1):436. doi: 10.1186/s12967-023-04276-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Zhang W, Wang N, et al. Thyroid Parameters and Kidney Disorder in Type 2 Diabetes: results from the METAL Study. J Diabetes Res. 2020;2020:4798947. doi: 10.1155/2020/4798947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Sun J, Xin S, Zhang X. Predictive Effects of FT3/FT4 on Diabetic Kidney Disease: an Exploratory Study on Hospitalized Euthyroid Patients with T2DM in China. Biomedicines. 2023;11(8):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W, Song D, Chen D, Duan W, Zhang J. The association of thyroid parameters with markers of chronic kidney disease in euthyroid patients with type 2 diabetes. Endocr J. 2023;70(7):687–696. doi: 10.1507/endocrj.EJ22-0643 [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Ding W, Wang H, Shi Y. Association Between Sensitivity to Thyroid Hormone Indices and Diabetic Retinopathy in Euthyroid Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndrome Obesity. 2023;16:535–545. doi: 10.2147/dmso.S399910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Sun J, Xu X, Xin S, Zhang X. The effect of Central and peripheral thyroid resistance indices on diabetic retinopathy: a study of hospitalized euthyroid patients with T2DM in China. Annals of Medicine. 2023;55(2):2249017. doi: 10.1080/07853890.2023.2249017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J, Xiang X, Qin Y, Gui J, Wan Q. Correlation of thyroid-related hormones with vascular complications in type 2 diabetes patients with euthyroid. Front Endocrinol. 2022;13:1037969. doi: 10.3389/fendo.2022.1037969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoja C, Xinaris C, Macconi D. Diabetic Nephropathy: novel Molecular Mechanisms and Therapeutic Targets. Front Pharmacol. 2020;11:586892. doi: 10.3389/fphar.2020.586892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuyama K, Ichiki T, Takeda K, et al. Downregulation of vascular angiotensin II type 1 receptor by thyroid hormone. Hypertension. 2003;41(3):598–603. doi: 10.1161/01.Hyp.0000056524.35294.80 [DOI] [PubMed] [Google Scholar]
- 30.Benedetti V, Lavecchia AM, Locatelli M, et al. Alteration of thyroid hormone signaling triggers the diabetes-induced pathological growth, remodeling, and dedifferentiation of podocytes. JCI Insight. 2019;4(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang G, Gao P, Zhao Z, et al. 3,5-Diiodo-l-thyronine ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. BBA. 2013;1832(5):674–684. doi: 10.1016/j.bbadis.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 32.Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and Kidney Disease: role of Oxidative Stress. Antioxid. Redox Signaling. 2016;25(12):657–684. doi: 10.1089/ars.2016.6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-Pituitary-Thyroid Axis. Compr Physiol. 2016;6(3):1387–1428. doi: 10.1002/cphy.c150027 [DOI] [PubMed] [Google Scholar]
- 34.Sagliocchi S, Cicatiello AG, Di Cicco E, et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019;24:101228. doi: 10.1016/j.redox.2019.101228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. doi: 10.1172/jci60047 [DOI] [PMC free article] [PubMed] [Google Scholar]