Abstract
Background:
Limited long-term safety data are published on HA/CaHA/L, a hybrid dermal filler combining hyaluronic acid (HA), calcium hydroxyapatite (CaHA), and lidocaine (L).
Methods:
This retrospective multicenter study assessed treatment-emergent adverse events (TEAEs) in adults treated with HA/CaHA/L. The full analysis set (FAS) included eligible consented adults (N = 403); the long-term safety analysis (LTSA) set included FAS participants with greater than or equal to 12-months HA/CaHA/L exposure (n = 243).
Results:
Participants were majority female (94.0%), with Fitzpatrick skin phototypes II/III (80.1%) and a mean age of 50.1 years. Most participants (86.4%) received one HA/CaHA/L treatment. The median time between participants’ first HA/CaHA/L treatment and chart review was 15.4 months. Participants received a mean of 2.2 mL (0.5-8.9 mL) filler per treatment. Treated areas were predominantly malar (71.2%) and mandible (69.7%) regions. Most participants (95.0%) had one or more aesthetic treatments other than HA/CaHA/L [eg, other dermal fillers (84.1%), botulinum toxin (63.3%)]. Nineteen (4.7%) FAS participants had 20 documented TEAEs; most (3.5%, n = 14 participants) were mild in severity. Twelve TEAEs in 11 participants (2.7%) were related to HA/CaHA/L: induration (three, 0.7%), edema (3, 0.7%), and implant site nodules (five, 1.2%), which were noninflammatory and likely related to product placement. Among the LTSA, 15 (6.2%) participants had 16 documented TEAEs (six edema, five implant site nodules, one inflammation, three skin induration, one hypersensitivity); most were mild in severity. Nine TEAEs in eight participants (3.3%) were HA/CaHA/L-related. No treatment-emergent serious AEs were reported.
Conclusion:
The data from this noninterventional retrospective study support the favorable longer term (>12 month) safety profile of HA/CaHA/L.
Takeaways
Question: What is the long-term safety profile of a hybrid hyaluronic acid and calcium hydroxyapatite filler (HA/CaHA/L)?
Findings: This retrospective, multicenter chart review in Brazil assessed HA/CaHA/L treatments (eg, number of treatments, injection instrument, treatment area) and other aesthetic treatments in adults who had received at least 1 HA/CaHA/L treatment. Adverse events occurred infrequently (<3%), were predominantly mild in severity, and resolved within 1 month.
Meaning: A hybrid soft tissue filler combining hyaluronic acid and calcium hydroxyapatite has a favorable safety profile that is comparable to that of other dermal fillers.
INTRODUCTION
Aging is associated with structural and functional changes in skin that lead to deterioration of skin quality (eg, wrinkles, laxity) and irregularities in skin tone and texture.1–5 Alterations in bone and soft tissue due to aging also occur. Changes in all layers of the face result in loss of facial volume, lack of smooth transitions between facial areas, and the appearance of facial laxity or sagginess.5–8 The use of dermal fillers allows for rapid restoration and augmentation of soft tissue, and a variety of dermal fillers are currently available. Commonly used soft tissue fillers contain hyaluronic acid (HA), which provides a rapid volumizing effect,9,10 or calcium hydroxyapatite, which has biostimulatory properties and stimulates neocollagenesis to produce a long-lasting lifting effect.9,11–13
HArmonyCa with Lidocaine (Allergan Aesthetics, an AbbVie company; Irvine, Calif.) is a hybrid dermal filler that combines crosslinked HA (20 mg/mL) gel matrix with calcium hydroxyapatite (CaHA; 55.7% w/w) microspheres and lidocaine (referred to hereafter as HA/CaHA/L) into a single, cohesive filler that does not require mixing or dilution before injection.14 The HA within HA/CaHA/L provides immediate volume and lift, whereas the CaHA stimulates the production of new collagen, resulting in dermal thickening and improvement in skin architecture, which increases skin firmness, improves overall skin quality, and produces a sustained lifting effect.15 The favorable safety profile of HA/CaHA/L has been demonstrated in three clinical trials,16 and the product is approved for use in multiple regions globally, including the European Union, Israel, Brazil, and South Africa. A recent prospective study in 15 participants showed that HA/CaHA/L treatment increased mid-face volume and skin viscoelasticity and was well tolerated during the 6-month follow-up period.17 However, limited published data are available on the long-term (≥12 months) safety of HA/CaHA/L.
This multicenter study conducted in Brazil retrospectively assessed the long-term safety of HA/CaHA/L, as measured by the incidence and severity of treatment-emergent adverse events (TEAEs), in adults with at least 12-months exposure to HA/CaHA/L.
MATERIALS AND METHODS
Study Design
This was a retrospective, noninterventional, chart review of adults who had previously received at least one injection of HA/CaHA/L (HArmonyCa with Lidocaine; Allergan Aesthetics, an AbbVie company, Irvine, Calif.) at six sites in Brazil. Investigators followed routine clinical practice for administration of HA/CaHA/L and were responsible for the selection of participants to be initially reviewed in this study; under their supervision, de-identified data from patients’ medical charts were reviewed by trained staff and entered into a validated electronic data capture system (CISIV Baseline Plus, version 3.2.2; Cisiv, Ltd, UK). Data were collected June to September 2022.
Participants
Eligible participants were adults (≥18 years) who had provided written informed consent and had received at least one injection of HA/CaHA/L. For each participant, data were abstracted from their medical chart from the date of first HA/CaHA/L use (on or after April 14, 2020) to the study period end date, which was defined as the date of ethics committee approval at each study site and ranged from June 14, 2022, to August 30, 2022.
Treatment Administration
Treatment data collected and analyzed for each participant included HA/CaHA/L administration, including treatment date(s), facial region(s) treated, side of face treated, injection volume, injection technique, and injection instrument and gauge used; medical history; aesthetic treatments and procedures concurrent to HA/CaHA/L; product complaints, incidence of adverse events (AEs); and medications and procedures used to treat and/or assess AEs. If applicable, information on pregnancy in female participants, including the date the site became aware of the pregnancy, the outcome of pregnancy (including due/delivery date), and any pregnancy-related AEs, was captured.
Safety Outcomes
The primary outcome measure was the number and percentage of participants who experienced at least one TEAE after 12-months or more exposure to HA/CaHA/L. Other safety outcomes included the incidence of treatment-emergent serious adverse events, the severity and relationship of a TEAE to the HA/CaHA/L product or injection procedure, the incidence of TEAEs as a result of device deficiencies, and medications and procedures used to treat and/or assess TEAEs. The severity of TEAEs was based on definitions from the Common Terminology Criteria for Adverse Events scale as follows: grade 1 (mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated), grade 2 (moderate; minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living), grade 3 (severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living), grade 4 (life-threatening consequences; urgent intervention indicated), and grade 5 (death related to TEAE).
Statistical Analyses
Statistical analyses on safety outcomes were conducted on two analysis populations: the full analysis set (FAS), which included all eligible, consented participants who had their medical chart data abstracted, and the long-term safety analysis set (LTSA), which included all participants in the FAS who also had 12 months or more of follow-up after treatment with HA/CaHA/L. Categorical data are summarized with frequency and percentage. Continuous data are summarized descriptively. Only complete data were used for the analyses, and no imputation was performed for missing data, except for missing or incomplete TEAE start/end dates. Where applicable, imputation methods were as follows. For missing or incomplete TEAE start dates, the first day of the year or first treatment date (if occurring in the same year) was imputed if only the year was available; the first day of the month or first treatment date (if occurring in the same month and year) was imputed if the month and year were available. For missing or incomplete TEAE end dates, the last day of the year or the study end date (if occurring in the same year) was imputed if only the year was available; the last day of the month or the study end date (if occurring in the same month and year) was imputed if the month and year were available. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, N.C.).
RESULTS
Participants
Of the 403 participants enrolled in the study and included in the FAS, 243 (60.3%) were included in the LTSA. Among the FAS (n = 403), the mean ± SD age of participants at the time of consent was 50.1 (10.79) years (range, 22–85 years). The majority of participants were female (94.0%, n = 379), White (91.8%, n = 370), and had Fitzpatrick skin phototypes II or III (80.1%). Three female participants (0.8%) had pregnancies documented during the study period. Among the LTSA (n = 243), participants had a mean ± SD age of 50.4 (10.64) years (range, 26–85 years). Most of the LTSA participants were female (94.7%, n = 230), White (90.5%, n = 220), and had Fitzpatrick skin phototypes II or III (80.2%). Demographic data are summarized in Table 1.
Table 1.
Participant Demographics
| FAS (n = 403) |
LTSA (n = 243) |
|
|---|---|---|
| Mean age, years (range) | 50.1 (22–85) | 50.4 (26–85) |
| Sex, n (%) | ||
| Female | 379 (94.0) | 230 (94.7) |
| Male | 24 (6.0) | 13 (5.3) |
| Fitzpatrick skin phototype, n (%) | ||
| I | 34 (8.4) | 20 (8.2) |
| II | 164 (40.7) | 101 (41.6) |
| III | 159 (39.5) | 94 (38.7) |
| IV | 40 (9.9) | 23 (9.5) |
| V | 5 (1.2) | 4 (1.6) |
| VI | 0 (0.0) | 0 (0.0) |
| Missing/not recorded | 1 (0.2) | 1 (0.4) |
| Female participants with pregnancies*, n (%) | 3 (0.8) |
Denominator is the number of female participants in the FAS (n = 379).
Most participants (95.0%, n = 383) had at least one aesthetic treatment or procedure documented during the study period, with the majority (53.1%, n = 214) having had four or more aesthetic treatments/procedures. Aesthetic treatments other than HA/CaHA/L received during the study period included other dermal fillers (84.1%, n = 339), botulinum toxin injections (63.3%, n = 255), microfocused ultrasound (23.6%, n = 95), and biostimulator fillers (14.1%, n = 57). Additionally, the majority of participants (83.9%, n = 338) received at least one other treatment or procedure on the same day that they were treated with HA/CaHA/L; the most frequent same-day treatment was another dermal filler (69.5%, n = 280), followed by botulinum toxin injections (33.8%, n = 136).
HA/CaHA/L Treatment Administration
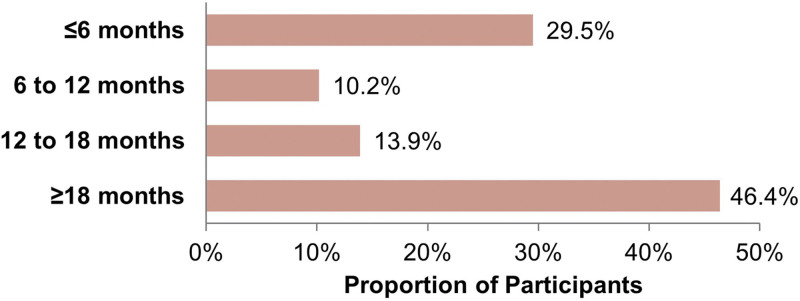
In the FAS, the majority of participants (86.4%, n = 348) were treated once with HA/CaHA/L over the study period; 10.2% (n = 41) received two treatments; 2.5% (n = 10) received three treatments; and 0.9% (n = 4) received four or more treatments. The median and mean ± SD time between participants’ first HA/CaHA/L treatment and medical chart review was 15.4 months and 14.50 (9.16) months (range, 0.1–27.4 months), respectively, and 46.4% (n = 187) of participants had been exposed to HA/CaHA/L for 18 months or more (Fig. 1). Participants received a mean of 2.2 mL (range, 0.5–8.9 mL) of HA/CaHA/L filler per visit, and a mean of 2.6 mL (range, 0.6–10.0 mL) over the entire study period. The most commonly treated facial regions were the malar (71.2%, n = 287) and mandible (69.7%, n = 281) regions, followed by the submalar region (32.8%, n = 132). Additional treatment areas were mentum/chin, marionette lines, nasolabial fold, prejowl, and others (Table 2). For participants who received HA/CaHA/L bilateral injections to the top treatment areas, the average filler volumes (Table 3) were 1.10 mL (malar region), 0.92 mL (submalar region), and 1.75 mL (mandible).
Fig. 1.
Time since first reported treatment with HA/CaHA/L. Data are from the full analysis set (N = 403).
Table 2.
HA/CaHA/L Treatment Summary
| FAS (N = 403) |
|
|---|---|
| Facial Regions Treated*, n (%) | |
| Malar region | 287 (71.2) |
| Mandible | 281 (69.7) |
| Marionette lines | 15 (3.7) |
| Mentum | 47 (11.7) |
| Nasolabial fold | 12 (3.0) |
| Submalar region | 132 (32.8) |
| Others | 58 (14.4) |
| No. Facial Regions Treated, n (%) | |
| 1 | 165 (40.9) |
| 2 | 88 (21.8) |
| 3 | 117 (29.0) |
| >3 | 33 (8.2) |
Regions treated on the same visit date were counted as a single HA/CaHA/L treatment.
Table 3.
Treatment Volume by Facial Region for Participants Receiving Bilateral HA/CaHA/L Injections
| Facial Region | |||
|---|---|---|---|
| Malar Region | Submalar Region | Mandible | |
| Participants, n (%) | 282 (70.0) | 130 (32.3) | 277 (68.7) |
| Volume, mL | |||
| Mean (SD) | 1.10 (0.68) | 0.92 (0.58) | 1.75 (0.96) |
| Median | 1.0 | 1.0 | 1.3 |
| Range | 0.2–5.0 | 0.2–4.25 | 0.2–5.0 |
Table lists injection volumes for the top treatment areas in participants who received HA/CaHA/L injections in the left and right sides of the face. The table does not include data on participants who received injections in only the right or only the left side of the face. Participants may have received HA/CaHA/L injections in multiple facial regions.
On average, participants were treated with HA/CaHA/L in 2.1 (SD = 1.07) different facial regions, though they were most commonly treated in only one facial region (40.9%, n = 165), followed by three regions (29.0%, n = 117), two regions (21.8%, n = 88), and more than three regions (8.2%, n = 33) (Table 2). HA/CaHA/L was almost always administered via cannula (99.8%) using retrograde (97.5%, n = 393) or fanning (52.9%, n = 213) injection techniques. For participants who received multiple HA/CaHA/L treatments during the study period (n = 55), the median time to the first retreatment was 296 days, the median time to a second retreatment (n = 14) was 202 days, and 231 days to a third retreatment (n = 4).
Safety
In the FAS, 19 (4.7%) participants had a total of 20 TEAEs documented during the study period (Table 4); the majority (n = 14 participants; 3.5%) were mild in severity and the remaining (n = 5 participants, 1.2%) were moderate in severity. Documented TEAEs included edema (n = 6), implant site nodule (n = 7), inflammation (n = 2), hypersensitivity (n = 1), and skin induration (n = 4). There were no reports of vascular complications or the Tyndall effect. Six participants (1.5%) had TEAEs that led to the initiation of medication or therapy. Of these, one participant (0.2%) with hypersensitivity was treated with steroids and antibiotics; one participant (0.2%) with inflammation was treated with steroids and hyaluronidase; three participants (0.7%) with edema were treated with antihistamines, antibiotics, steroids, and/or hyaluronidase; and one participant (0.2%) with edema had resolution after initiation of medication (antihistamine and steroid), microfocused ultrasound, radiofrequency treatment, and partial removal of the HA/CaHA/L via ultrasound-guided administration of hyaluronidase. Twelve TEAEs occurring in 11 participants (2.7%) were deemed related to the HA/CaHA/L product and/or procedure; all except one TEAE were mild. Of these, five (1.2%) participants reported implant site nodules that occurred in the mandible, prejowl, and/or submalar region; three (0.7%) participants reported edema that occurred in the mandible, malar, and submalar regions; and four (1.0%) participants reported skin induration that occurred in the mandible and malar regions. Five TEAEs (implant site nodule) occurring in five participants (1.2%) were deemed related to the procedure; the investigational sites confirmed these nodules were noninflammatory, mild in severity, had early onset, and were deemed to be product accumulation. Three of the HA/CaHA/L-related implant site nodules resolved by study end; of these, two resolved with massage alone and one resolved without intervention. The two nodules that had not resolved by database lock were confirmed to have resolution after study end by the investigators.
Table 4.
Summary of Treatment-emergent AEs
| Relationship to HA/CaHA/L | TEAE | Location | Severity |
|---|---|---|---|
| Related | Edema | Malar region | Mild |
| Malar region, submalar region, mandible | Moderate | ||
| Malar region, mandible* | Mild | ||
| Skin induration | Mandible* | Mild | |
| Mandible | Mild | ||
| Mandible | Mild | ||
| Malar region | Mild | ||
| Implant site nodule | Submalar region | Mild | |
| Prejowl | Mild | ||
| Prejowl | Mild | ||
| Prejowl | Mild | ||
| Mandible, prejowl | Mild | ||
| Not related | Edema | Marionette lines | Mild |
| Periorbital | Mild | ||
| Periorbital | Moderate | ||
| Inflammation | Upper lip (mucosal/red), mentum/chin | Mild | |
| Malar region, mentum/chin, mandible, periorbital | Moderate | ||
| Implant site nodule | Prejowl | Mild | |
| Jowl | Moderate | ||
| Hypersensitivity | Upper and lower lip (mucosal/red) | Moderate |
Each line of the table represents a single TEAE in a single participant.
TEAEs reported for the same participant.
The duration of TEAEs in the FAS ranged from less than 1 month to 12 months. All TEAEs but two were documented as resolved by study end, though investigators confirmed resolution of these remaining TEAEs after database lock. No participants in the FAS experienced TEAEs of grades 3, 4, or 5 severity, and no participant had a documented TESAE. Among the three participants who had documented pregnancies during the study period, there were no pregnancy-related AEs reported.
Safety outcomes were similar among the LTSA, with 15 participants (6.2%) having a total of 16 documented TEAEs; the majority (n = 10 participants; 4.1%) were mild in severity. Six (2.5%) participants reported edema that was mild (n = 4, 1.6%) or moderate (n = 2, 0.8%) in severity. Five (2.1%) participants reported implant site nodules that were mild (n = 4, 1.6%) or moderate (n = 1, 0.4%) in severity. One (0.4%) participant reported inflammation that was moderate in severity, three (1.2%) participants reported skin induration that was mild in severity, and one (0.4%) participant experienced hypersensitivity that was moderate in severity. Nine TEAEs in eight participants (3.3%) were deemed related to the HA/CaHA/L product and/or injection procedure. Six participants (2.5%) had TEAEs that led to a medication or therapy being initiated; details are provided above, as LTSA participants are a subset of the FAS group. Among these, one (0.4%) participant experienced a TEAE that led to partial removal of HA/CaHA/L via ultrasound-guided hyaluronidase administration, and two (0.8%) participants had one TEAE each that required a procedure to be performed.
DISCUSSION
This multicenter, noninterventional, retrospective chart review shows that HA/CaHA/L has a favorable long-term (≥12 months) safety profile, as measured by a low (< ~3%) incidence of TEAEs. The majority of TEAEs experienced by participants were mild in severity and were of classifications that can be expected with all dermal fillers (eg, edema, implant site nodule, skin induration).
The majority of participants received a single HA/CaHA/L treatment during the course of the study period. A smaller subset received two or three treatments. HA/CaHA/L was predominantly administered to one facial region, and most commonly targeted the malar region and mandible, though approximately 30% of HA/CaHA/L injections targeted the submalar region. The mean volume of HA/CaHA/L injection per treatment, as well as the mean total received during the study, was approximately 2 mL. Most injections were carried out using a retrograde injection technique with a cannula. Aesthetic treatments performed and documented during the study period were not limited to HA/CaHA/L, as almost all participants received more than one non-HA/CaHA/L treatment or procedure, with the majority having received at least four treatments. These other treatments were primarily other injectable fillers (ie, HA-only fillers, biostimulator fillers) and botulinum toxin injections.
HA/CaHA/L treatment was well tolerated, as evidenced by the low incidence of TEAEs in both the FAS and in participants with 12 months or more exposure to HA/CaHA/L (LTSA). The majority of TEAEs experienced by participants were mild in severity, occurred at rates similar to those reported for HA or CaHA dermal fillers, and were of classifications that can be expected with all dermal fillers.16–20 Importantly, few participants (2.5%) experienced TEAEs that required intervention with medication or a procedure.
Of the TEAEs in the FAS that were determined to be related to HA/CaHA/L, the majority (nine of 12) occurred within 1 month of treatment. HA/CaHA/L-related reports of edema experienced by three participants occurred in the malar region (three events), submalar region (one event), and mandible (two events). Note that one participant experienced edema in all three regions treated with HA/CaHA/L, and one participant experienced edema in two of the five regions in which they received HA/CaHA/L. These events of edema occurred 0 to 6 months after injection and resolved with treatment (ie, antibiotics, antihistamines, steroids, or partial removal of the filler) in less than 4 months. Treatment-related reports of skin induration experienced by three participants occurred in the mandible (three events) and malar region (one event); these events occurred 0 to ~2 weeks after injection, and most resolved within ~1 month, with one event resolving in 7 months. Treatment for skin induration included massage in two participants. Treatment-related reports of implant site nodules experienced by five participants occurred in the mandible (one event), prejowl area (four events), and submalar region (one event). All treatment-related reports of implant site nodules were noninflammatory and had an onset of 0 to 2 months after injection. Of the three nodules with confirmed resolution by study end, nodules resolved after 0 to 4 months. Treatment for nodules included massage in three participants. Ultrasound examination conducted in one participant who had a nodule with onset 3 weeks postinjection showed the cause to be product accumulation.
In the opinion of the authors, means of limiting implant site nodules post-HA/CaHA/L injection include use of slow retrograde technique, avoidance of bolus injections, cessation of injection prior to removal of the cannula (thus avoiding superficial deposition of product), and delivering all injections to the deep dermal or subdermal layers per the instructions for use. The authors recommend injecting smaller volumes near the cannula entry point when employing a fanning technique to avoid the potential for product accumulation at this pivot point. Furthermore, it is recommended to avoid large injection volumes in a single area or in hyperdynamic areas of the face (eg, prejowl) where movement of the depressor anguli oris muscle can lead to accumulation of product even when injected correctly. All patients should be followed up in 15 to 20 days to correct possible accumulations through massage.
Examination of other aesthetic treatments (eg, HA fillers) received at the time of, or after, HA/CaHA/L treatment did not reveal any non-HA/CaHA/L treatments that may have contributed to or caused these related TEAEs. Given the small number of TEAEs related to the HA/CaHA/L product and/or procedure, it is difficult to identify potential contributing factors (eg, concurrent aesthetic treatments, facial region treated, volume of HA/CaHA/L, number of HA/CaHA/L treatments, injection technique/instrument). Importantly, 10 of the 12 TEAEs related to HA/CaHA/L had confirmed resolution by study end. There were no pregnancy-related AEs reported among the three female participants who had pregnancies documented during the study period.
Investigators noted that there was a learning curve with HA/CaHA/L injections, but that its behavior was similar to that of CaHA- and HA-only fillers, allowing them to apply their knowledge of injecting other dermal fillers to HA/CaHA/L. Given similarities in the behavior and injection techniques with HA/CaHA/L compared with other dermal fillers, many AEs are likely related to the incorrect application of the product, such as compromised or inadequate asepsis, bolus injections, and large volume injections in highly mobile facial areas. Thus, attention to technique will limit many potential AEs. The learning curve with HA/CaHA/L also encompassed which facial regions were treated; experience with the product led investigators to understand that treating the lateral face allows for safer/simpler injections and optimal outcomes in terms of the product’s lifting effects, which would be further enhanced by its biostimulatory properties.
Limitations
There are inherent limitations with any study using a retrospective design, such as the completeness and accuracy of available data potentially differing across investigational sites. There is the possibility that existing medical record data do not contain all information required to address study objectives, and participants may not have attended the recommended follow-up visits or may have presented with a complaint to an alternative provider, which may lead to an underestimation of the rate of AEs. Additionally, the investigators in this study are highly experienced injectors, which may have led to an underestimation of TEAEs associated with HA/CaHA/L, especially those related to the injection or procedure itself; however, the AE profile in this study is consistent with that of prior clinical trials.16 Finally, as any patient treated with HA/CaHA/L during the study period was included, there may be a lack of homogeneity among the patient group.
CONCLUSION
The data from this multicenter, noninterventional, retrospective chart review support the favorable long-term (>12 months) safety profile of this hybrid dermal filler in real-world clinical practice.
DISCLOSURE
Braz is an investigator and advisory board member for Allergan Aesthetics, an AbbVie company. Colucci is an investigator, speaker, and consultant for Allergan Aesthetics, an AbbVie company. Macedo de Oliveira, Monteiro, Ormiga, and Wanick are investigators, speakers, and advisory board members for Allergan Aesthetics, an AbbVie company. Cazerta, Kerson, Musumeci, and Silberberg are full-time employees of AbbVie Inc and may own AbbVie stock. Allergan Aesthetics, an AbbVie Company, funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Sarah J. Cross, PhD, of AbbVie Inc. and editorial support was provided by Angela T. Hadsell of AbbVie Inc.; both were funded by AbbVie Inc.
Footnotes
Published online 12 February 2024.
Presented at the 2023 Annual Meeting of the Sociedade Brasileira de Cirurgia Dermatologica (SCBD), the 2023 Societatea Română de Dermatologie (SRD) National Congress of Dermatology meeting, and the 2023 International Caucasian Congress on Plastic Surgery and Dermatology Kolkhida Meeting.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Haydont V, Bernard BA, Fortunel NO. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev. 2019;177:150–156. [DOI] [PubMed] [Google Scholar]
- 2.Okano Y, Masaki H, Sakurai H. Dysfunction of dermal fibroblasts induced by advanced glycation end-products (AGEs) and the contribution of a nonspecific interaction with cell membrane and AGEs. J Dermatol Sci. 2002;29:171–180. [DOI] [PubMed] [Google Scholar]
- 3.Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23:996–1001. [DOI] [PubMed] [Google Scholar]
- 4.Choi JW, Kwon SH, Huh CH, et al. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013;19:e349–e355. [DOI] [PubMed] [Google Scholar]
- 5.Swift A, Liew S, Weinkle S, et al. The facial aging process from the “inside out”. Aesthet Surg J. 2021;41:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592–600. [DOI] [PubMed] [Google Scholar]
- 7.Rohrich RJ, Pessa JE, Ristow B. The youthful cheek and the deep medial fat compartment. Plast Reconstr Surg. 2008;121:2107–2112. [DOI] [PubMed] [Google Scholar]
- 8.Boehm LM, Morgan A, Hettinger P, et al. Facial aging: a quantitative analysis of midface volume changes over 11 years. Plast Reconstr Surg. 2021;147:319–327. [DOI] [PubMed] [Google Scholar]
- 9.Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41:S373–S381. [DOI] [PubMed] [Google Scholar]
- 10.de la Guardia C, Virno A, Musumeci M, et al. Rheologic and physicochemical characteristics of hyaluronic acid fillers: overview and relationship to product performance. Facial Plast Surg. 2022;38:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunha MG, da, Engracia M, de Souza LG, et al. Bioestimuladores e seus mecanismos de ação. Surg Cosmet Dermatol. 2020;12:109–117. [Google Scholar]
- 12.González N, Goldberg DJ. Evaluating the effects of injected calcium hydroxylapatite on changes in human skin elastin and proteoglycan formation. Dermatol Surg. 2019;45:547–551. [DOI] [PubMed] [Google Scholar]
- 13.Zerbinati N, Calligaro A. Calcium hydroxylapatite treatment of human skin: evidence of collagen turnover through picrosirius red staining and circularly polarized microscopy. Clin Cosmet Investig Dermatol. 2018;11:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allergan Aesthetics. HArmonyCa Lidocaine. Instructions for use. 2021. [Google Scholar]
- 15.Braz A, Kutikov A, Pierce A, et al. Biological response of the combination filler, HArmonyCaTM, in a rodent model. Poster presented at: Aesthetics & Anti-Aging Medicine World Congress Annual Meeting; Mar 31–Apr 2, 2022; Monte Carlo, Monaco. [Google Scholar]
- 16.Allergan Aesthetics Data on File: REF-83821. [Google Scholar]
- 17.Urdiales-Gálvez F, Braz A, Cavallini M. Facial rejuvenation with the new hybrid filler HArmonyCa: Clinical and aesthetic outcomes assessed by 2D and 3D photographs, ultrasound, and elastography. J Cosmet Dermatol. 2023;22:2186–2197. [DOI] [PubMed] [Google Scholar]
- 18.Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152–161. [DOI] [PubMed] [Google Scholar]
- 19.Signorini M, Liew S, Sundaram H, et al. ; Global Aesthetics Consensus Group. Global Aesthetics Consensus: Avoidance and management of complications from hyaluronic acid fillers—evidence and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137:961e–971e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipp-Dormston WG, Goodman GJ, De Boulle K, et al. Global approaches to the prevention and management of delayed-onset adverse reactions with hyaluronic acid-based fillers. Plast Reconstr Surg Glob Open. 2020;8:e2730. [DOI] [PMC free article] [PubMed] [Google Scholar]