Abstract
Tobacco products generally contain tobacco-derived nicotine (TDN; having ~99+% (S)-(−)-nicotine). Recent United States regulation has led some producers to transition to synthetic (“tobacco-free”) nicotine. For example, Puff Bar is now marketed with tobacco-free nicotine (TFN; presumed to be racemic). To evaluate the claim that these new products contain TFN, we evaluated the presence of the two nicotine optical isomers by 1H NMR spectroscopy, polarimetry, and gas chromatography–mass spectrometry. Older Puff Bars were found to contain (S)-(−)-nicotine, and newer “TFN” Puff Bars were found to contain both (R)-(+) and (S)-(−) isomers—indicating TFN, albeit with slightly more of the (S)-(−)-nicotine form.
Graphical Abstract
Nicotine has been primarily obtained by extraction from the tobacco plant (Nicotiana tabacum), although e-cigarette brands such as Puff Bar claim to have transitioned to synthetic nicotine.1 Nicotine extracted from the tobacco plant is almost entirely in the (S)-(−)-nicotine form.2 Synthetic nicotine would generally contain both isomers: (R)-(+)- and (S)-(−)-nicotine, produced in equal quantities during synthesis giving the racemic mixture. While it is possible to separate the (R)-(+) and (S)-(−) forms or produce enrichment of the desired enantiomer by chiral synthesis, this would add considerably to the cost, and so to our knowledge this is not generally done. It has been reported that (S)-(−)-nicotine is more toxic than (R)-(+)-nicotine in multiple species and can produce differing degrees of side effects.3,4 The racemic (R,S)-(±)-nicotine mixture has been reported to be more toxic than (R)-(+)-nicotine.4 The two nicotine isomers have also been reported to induce different levels of acetylcholinesterase inhibition, with (R)-(+)-nicotine being more potent.5 The binding affinities of the two nicotine isomers to nicotinic acetylcholine receptors have been estimated to differ by 10-fold, where (S)-(−)-nicotine has the greater affinity.6 Further, nicotine consumers (i.e., of cigarettes) titrate their nicotine dose to induce satiety.7 E-cigarette consumers have been found to increase their vaping when provided with lower nicotine concentration products compared to higher nicotine concentration products.8 Much of this work regarding consumer nicotine titration behavior has been based on natural nicotine, which is primarily the (S)-(−) form. The effects of the shift from primarily (S)-(−)-nicotine consumption toward mixed (R,S)-(±)-nicotine consumption in e-liquids on human health and behavior are not fully understood and warrant further study.
Hellinghausen et al. (2017) reported that a small selection of tobacco-free nicotine (TFN) e-liquids available at the time contained 50/50 (S)-(−)-nicotine and (R)-(+)-nicotine.9 Herein, we use measurements of the relative content of the (R)-(+)- and (S)-(−)-isomers of nicotine to evaluate the recent claim by Puff Bar that their latest disposable e-cigarette products contain TFN, as well as to compare the currently available Puff Bars with their previously available devices that did not indicate this (and so can be presumed to be made with tobacco-derived nicotine (TDN)). The presence of (R)-(+)-nicotine and (S)-(−)-nicotine was identified in Puff Bar e-liquids using three different methods: 1H NMR spectroscopy, polarimetry, and gas chromatography–mass spectrometry (GC–MS). NMR spectroscopy and GC–MS are common techniques that have been employed by us and others to analyze e-liquid compositions, e-cigarette aerosol chemistry, and the protonation state of nicotine in e-liquids, while polarimetry has not been commonly used for e-liquid analysis.10–14
Puff Bars labeled to contain 5% nicotine were purchased from online retailers and from local e-cigarette shops in Portland, OR. Some of the Puff Bar e-cigarettes stated that they contained “tobacco-free nicotine” (i.e., synthetic nicotine, which would be racemic: (R,S)-(±)-nicotine), while the older Puff Bar e-cigarettes did not make this claim and presumably contained tobacco-derived nicotine (i.e., ~99+% (S)-(−)-nicotine).
(R)-(−)-1,1′-Binaphthyl-2,2′-diyl hydrogen phosphate (BNPPA) was used as an NMR chiral complexing agent based on its use by Ravard and Crooks.15 NMR samples were prepared by adding nicotine to the NMR solvent, DMSO-d6, and combining aliquots of this mixture with varying quantities of BNPPA. A 600 MHz Bruker AVANCE III NMR spectrometer was used for data collection. Spectra were collected at 25 °C using 16 scans (NS) with a 30° observation pulse and a relaxation delay of 3 s.
For polarimetry testing, nicotine solutions and Puff Bar e-liquids were diluted in ethanol. Optical rotations were measured, and specific rotations were calculated for each sample using Biot’s law (Table S2).
An evaluation of the nicotine stereoisomers was also performed using a solid phase microextraction (SPME) GC–MS method. The retention times for (S)-(−)-nicotine and (R)-(+)-nicotine were 2369 and 2380 s, respectively.
1H NMR data were compared for Puff Bar e-liquids in flavors Cool Mint (Figure 1) and Strawberry Watermelon/Straw Watermelon (Figure S1). Older e-liquids in each flavor did not mention the origin of the nicotine and were assumed to contain TDN, which were expected to be 99+% (S)-(−)-nicotine. The addition of BNPPA to each e-liquid resulted in a single set of BNPPA-bound nicotine peaks (Figure 1A,B; Figure S1A,B), indicating that only one form of nicotine ((S)-(−)-nicotine) was present. Newer Puff Bar e-liquids in each flavor labeled as containing TFN were similarly tested. The addition of BNPPA to these e-liquids resulted in the separation of some of the (R)-(+)- and (S)-(−)-nicotine peaks (Figure 1C,D; Figure S1C,D). In particular, nicotine protons Ha and He had distinct resonances for the (R)-(+)- and (S)-(−)-nicotine forms (Figure 1D; Figure S1D). The ratio of (R)-(+)- to (S)-(−)-nicotine can be determined by integrating the and resonances. As can be visually observed in the expansions in Figure 1D and Figure S1D, slightly more (S)-(−)-nicotine (integral ratios ~1.0:1.2 ± 0.1) was determined to be present in both TFN e-liquid flavors. This integral ratio is 1.0:1.0 for authentic racemic nicotine (Figure S2). Additionally, the benzoic acid/nicotine ratios and nicotine concentrations for Puff Bar e-liquids were analyzed; some were found to be similar to JUUL, with a ratio of ~1,12 and others were as high as 1.3 (Table S1). Free-base nicotine content (αfb) ranged from 0.07 to 0.19 (Table S1). For all flavors tested (Table S1), αfb was found to range from 0.07 to 0.19. Differences in αfb are likely due to different acid/nicotine ratios as well as the presence of different flavorants, some of which can be acidic and thus influence the acid/base chemistry of these products.13
Figure 1.
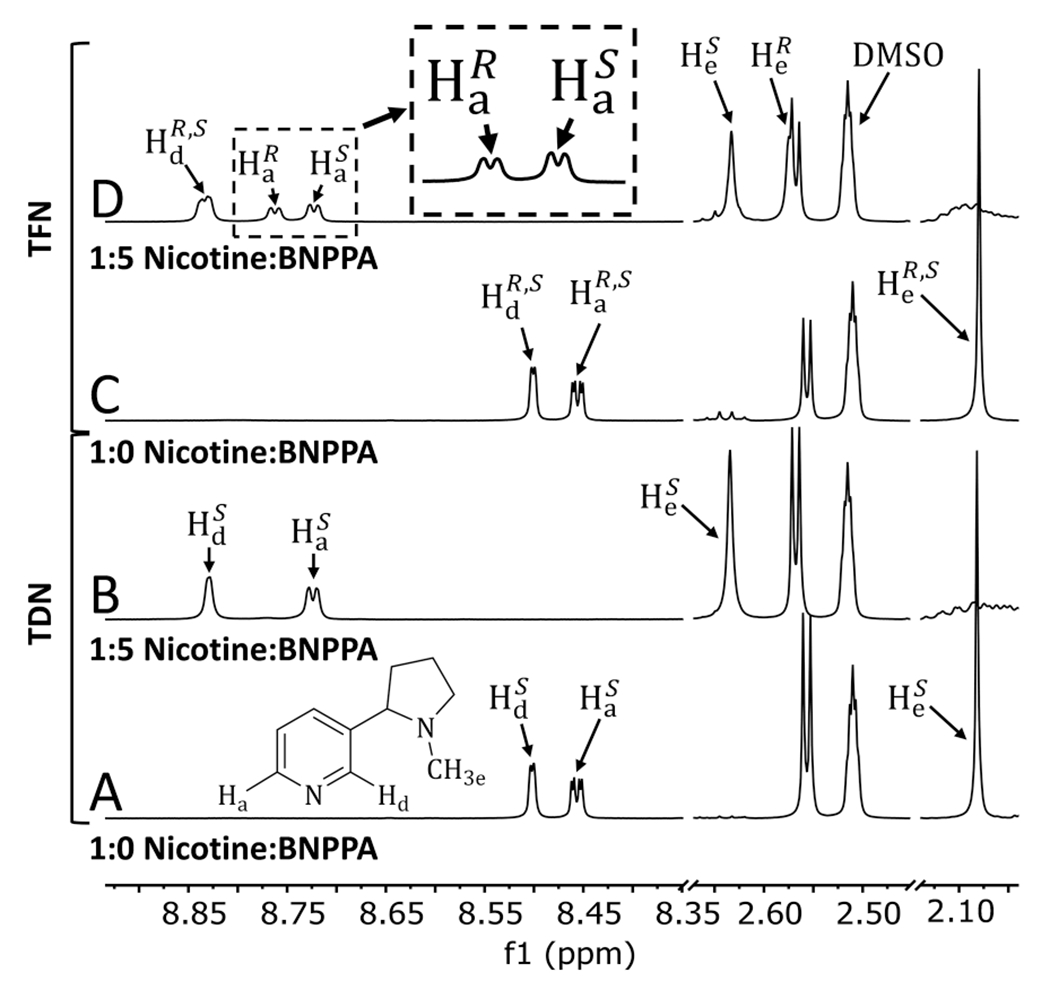
1H NMR spectra for Puff Bar e-liquids in flavor “Cool Mint” without and with (R)-(−)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate (BNPPA). An e-liquid presumed to be tobacco-derived nicotine (TDN) is shown in spectra A and B without and with BNPPA, respectively. An e-liquid advertised as containing tobacco-free nicotine (TFN) is shown in spectra C and D without and with BNPPA, respectively. For TDN, no splitting of protons Ha or He is observed confirming that only one form of nicotine, (S)-(−)-nicotine, is present. For TFN, the addition of BNPPA results in distinct peaks appearing for the (R)-(+) and (S)-(−) forms of nicotine for protons Ha and He. and integrate ~1:1.2 ± 0.1, respectively, which represents molar equivalents.
The same Puff Bar e-liquids were also analyzed using polarimetry, and similar chirality results were obtained. (S)-(−)-Nicotine and (R)-(+)-nicotine are optically active alkaloids with a specific rotation () of −169.0° and +169.0°, respectively.6,16 Tobacco products and other alkaloids have been analyzed with a polarimeter to determine the enantiomeric excess and from the measured optical rotation (α) of the sample.17 Polarimeter measurements are usually accompanied by NMR spectroscopy or GC-–MS analysis to obtain more accurate results for the enantiomeric ratios.6,18 E-liquids have not been analyzed with a polarimeter to date. E-liquids with TDN should result in a levorotation, similar to tobacco products in the past.17 However, e-liquids containing TFN would result in an α of 0.0° because the sample is racemic, resulting from a synthesis of nicotine, giving equal quantities of (R)-(+)- and (S)-(−)-nicotine.
The TFN samples with and without t-butylamine had measured α values at or near 0.0° supporting our hypothesis that these e-liquids contain racemic nicotine (Table S2). However, the observed optical rotations were slightly levorotatory, perhaps resulting from the presence of chiral flavorants or a slight excess of (S)-(−)-nicotine, as seen in the NMR data above. The e-liquids with TDN with and without t-butylamine rotated the plane of light counterclockwise much more than the TFN e-liquids since the samples contained mostly (S)-(−)-nicotine. The nicotine concentrations were estimated in the TDN e-liquids with the linear regression and were more accurate when calculated with the measurements from the TDN e-liquids with t-butylamine (Table S2 and Figure S4). The TDN e-liquids contained nicotine salts (protonated nicotine), which are dextrorotatory and can result in erroneous conclusions unless accounted for.19
Puff Bar TDN and TFN e-liquids were finally analyzed by GC–MS, and the data agree with the results obtained by 1H NMR and polarimetry: older Puff Bar e-liquids contained mainly (S)-(−)-nicotine, and the newer products advertised as TFN contained a mixture of both (R)-(+)- and (S)-(−)-nicotine; the peak intensity peak ratio (S/R) was about 12% larger for the TFN e-cigarette samples than for the authentic racemic nicotine (Figure S5). The reason for this is unknown, but possibilities include the residual contamination of the equipment used to produce commercial nicotine or the addition of TDN to synthetic nicotine.
In summary, we found that the Puff Bar e-liquids we tested that were advertised to be tobacco-free did contain both (R)-(+) and (S)-(−) forms of nicotine as evaluated by 1H NMR spectroscopy, polarimetry, and GC–MS. The ratio of (R)-(+)- to (S)-(−)-nicotine was found to be ~1:1.2 for both Cool Mint and Strawberry Watermelon flavors, as determined by 1H NMR spectroscopy for which we had the highest degree of peak separation. The expected ratio of (R)-(+)- to (S)-(−)-nicotine was 1:1 because the synthesis of nicotine would produce the racemic mixture (equimolar quantities of the (R)-(+) and (S)-(−) forms). Puff Bar e-liquids not advertised to be tobacco-free were found to contain (S)-(−)-nicotine, and (R)-(+)-nicotine was not identified, which is consistent with tobacco-derived nicotine.
Supplementary Material
ACKNOWLEDGMENTS
We thank R.M. Strongin for useful discussions about this topic.
Funding
This work was supported by the U.S. National Institutes of Health, grant R01ES025257. Research reported was supported by the NIEHS and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
ABBREVIATIONS
- TFN
tobacco-free nicotine
- TDN
tobacco-derived nicotine
- α
optical rotation
specific rotation
- GC–MS
gas chromatography–mass spectrometry
- NMR spectroscopy
nuclear magnetic resonance spectroscopy
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemrestox.1c00192.
Experimental details, as well as additional NMR, GC–MS, and polarimetry data and information (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrestox.1c00192
Contributor Information
Anna K. Duell, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
Paul J. Kerber, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
Wentai Luo, Department of Chemistry and Department of Civil & Environmental Engineering, Portland State University, Portland, Oregon 97207-0751, United States.
David H. Peyton, Department of Chemistry, Portland State University, Portland, Oregon 97207-0751, United States
REFERENCES
- (1).Maloney J. Puff Bar Defies FDA Crackdown on Fruity E-Cigarettes by Ditching the Tobacco. Wall Street Journal. March 2, 2021. https://www.wsj.com/articles/puff-bar-defies-fda-crackdown-on-fruity-e-cigarettes-by-ditching-the-tobacco-11614681003; Vol. 2021. [Google Scholar]
- (2).Benowitz NL, Hukkanen J, and Jacob P 3rd. (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol 192, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Barlow RB, and Hamilton JT (1965) The stereospecificity of nicotine. Br. J. Pharmacol. Chemother 25 (1), 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yildiz D, Ercal N, and Armstrong DW (1998) Nicotine enantiomers and oxidative stress. Toxicology 130 (2–3), 155. [DOI] [PubMed] [Google Scholar]
- (5).Yang J, Chen Y, Liu Z, Yang L, Tang J, Miao M, Gan N, and Li H (2019) Differences between the binding modes of enantiomers S/R-nicotine to acetylcholinesterase. RSC Adv. 9 (3), 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Pogocki D, Ruman T, Danilczuk M, Danilczuk M, Celuch M, and Walajtys-Rode E (2007) Application of nicotine enantiomers, derivatives and analogues in therapy of neurodegenerative disorders. Eur. J. Pharmacol 563 (1–3), 18. [DOI] [PubMed] [Google Scholar]
- (7).Benowitz NL In Risks Associated with Smoking Cigarettes with Low Machine- Measured Yields of Tar and Nicotine; Burns DM, Ed.; U.S. Department of Health and Human Services, 2001. [Google Scholar]
- (8).Dawkins LE, Kimber CF, Doig M, Feyerabend C, and Corcoran O (2016) Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (BPsychopharmacology (Berl) 233 (15–16), 2933. [DOI] [PubMed] [Google Scholar]
- (9).Hellinghausen G, Lee JT, Weatherly CA, Lopez DA, and Armstrong DW (2017) Evaluation of nicotine in tobacco-free-nicotine commercial products. Drug Test. Anal 9 (6), 944. [DOI] [PubMed] [Google Scholar]
- (10).Duell AK, McWhirter KJ, Korzun T, Strongin RM, and Peyton DH (2019) Sucralose-Enhanced Degradation of Electronic Cigarette Liquids during Vaping. Chem. Res. Toxicol 32 (6), 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Duell AK, Pankow JF, and Peyton DH (2018) Free-Base Nicotine Determination in Electronic Cigarette Liquids by 1H NMR Spectroscopy. Chem. Res. Toxicol 31 (6), 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Duell AK, Pankow JF, and Peyton DH (2019) Nicotine in tobacco product aerosols: ’It’s deja vu all over again. Tob Control 29 (6), 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Pankow JF, Duell AK, and Peyton DH (2020) Free-Base Nicotine Fraction αfb in Non-Aqueous versus Aqueous Solutions: Electronic Cigarette Fluids Without versus With Dilution with Water. Chem. Res. Toxicol 33 (7), 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, and Peyton DH (2017) Benzene formation in electronic cigarettes. PLoS One 12 (3), No. e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ravard A, and Crooks PA (1996) Chiral purity determination of tobacco alkaloids and nicotine-like compounds by 1H NMR spectroscopy in the presence of 1, 1′-binaphthyl-2, 2′-diylphosphoric acid. Chirality 8 (4), 295. [Google Scholar]
- (16).Nicotine. https://pubchem.ncbi.nlm.nih.gov/compound/nicotine#section=Spectral-Information; Vol. 2021.
- (17).Perfetti TA, and Swadesh JK (1991) On-Line Determination of the Optical Purity of Nicotine. Journal of Chromatography A 543, 129. [Google Scholar]
- (18).Li X-C, Liu F, Su H-G, Guo L, Zhou Q-M, Huang Y-J, Peng C, and Xiong L (2019) Two pairs of alkaloid enantiomers from Ganoderma luteomarginatum. Biochem. Syst. Ecol 86, 103930. [Google Scholar]
- (19).Gorrod JW, and Jacob P, Eds. Analytical Determination of Nicotine and Related Compounds and Their Metabolites; Elsevier: Amsterdam, 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


