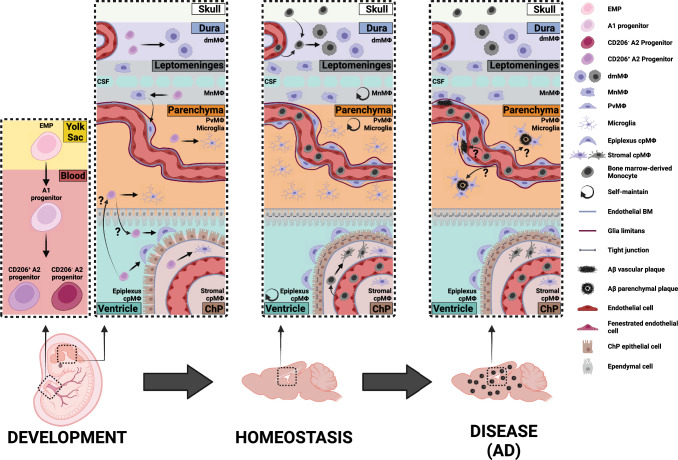
Fig. 1.
CNS resident macrophages during development, homeostasis, and Alzheimer’s disease (AD). During embryogenesis, erythromyeloid precursors (EMP) from the yolk sac differentiate to an intermediate immature population (A1) which transition into pre-macrophages progenitors (A2). These immature macrophages migrate through the developing blood vessels and start to invade the neural tube by E9.5. While some CD206+ A2 progenitors seed the parenchyma to become microglia, others engraft 1) the dura to become dural macrophages (dmMΦ), 2) the choroid plexus (ChP) to become epiplexus or stromal choroid plexus macrophages (cpMΦ), and 3) the leptomeninges to become leptomeningeal macrophages (MnMΦ). MnMΦ populate the perivascular spaces postnatally, differentiating into perivascular macrophages (PvMΦ). The PvMΦ are sandwiched between the endothelial basement membrane (BM) and the glia limitants. In the developed CNS, microglia, MnMΦ, PvMΦ, and epiplexus cpMΦ are long-lived and self-maintain. Instead, dmMΦ and stromal cpMΦ are slowly replaced by bone marrow-derived Monocytes. During AD context, not only microglia, but also PvMΦ and MnMΦ have a critical role in the clearance of vascular and parenchymal amyloid beta (Aβ) plaques. The maintenance of blood–brain barrier integrity, along with the possible contribution of monocyte-derived cells in clearing plaques, remains unclear