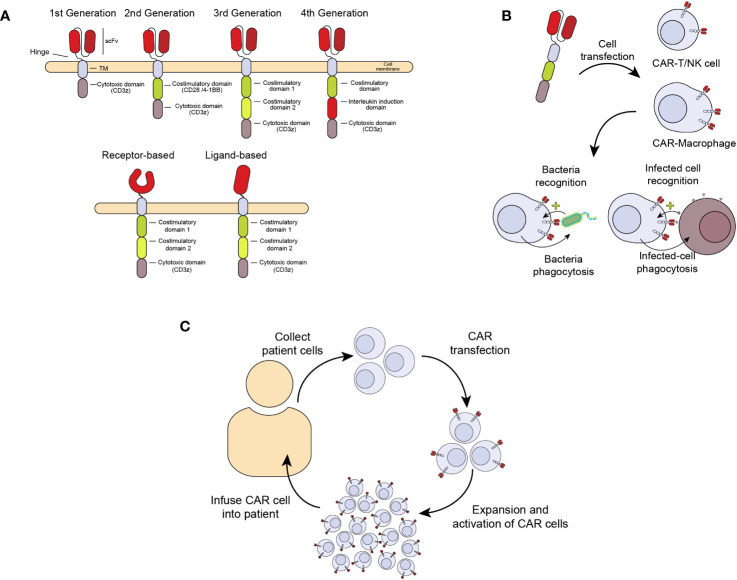
Figure 1.
Generation of chimeric antigen receptors (CAR) expressed in cells from the immune system with antimicrobial activity. (A) Chimeric receptor involves an antibody fragment (scFv) or receptor against an antigen followed by a hinge peptide, a transmembrane domain, and one or more intracellular domains of proteins like the CD3 zeta (ζ) chain of the T-cell receptor complex (CD3ζ) and CD28 or 4-1BB (CD137), with the ability to signal and induce proliferation, activation, and cytotoxicity in the cell expressing the CAR. (B) Once the extracellular scFv/receptor domain of the CAR recognizes the antigen in an infected cell, the CAR cell gets activated and eliminates the infected cells. (C) CAR cell production. The manufacturing process begins with collecting peripheral blood mononuclear cells from a patient by leukapheresis. The cells are enriched, selected, and activated, followed by transduction with a self-inactivating lentiviral vector containing the CAR transgene. Cells are expanded following transduction until the final product dose requirements are met. Once the patient is ready, the cells are infused into the same patient who provided the leukapheresed cells.