Visual Abstract
Abstract
Secondary central nervous system (CNS) lymphoma (SCNSL) is a rare but clinically challenging scenario with historically disappointing outcomes. SCNSL refers to lymphoma that has spread into the CNS concurrently with systemic disease or CNS relapse during or after frontline immunochemotherapy, presenting with or without systemic lymphoma. Diffuse large B-cell lymphoma (DLBCL) denotes the most common entity, but an increased incidence is observed in other histologies, such as Burkitt lymphoma and mantle-cell lymphoma. The incidence, timing in disease course, location, evidence supporting the use of CNS prophylaxis, and treatment pathways vary according to histology. No randomized data exist to delineate the best treatment approaches with current recommendations based on retrospective and single-arm studies. However, a regimen comprising immunochemotherapy, incorporating agents that cross the blood-brain barrier, followed by thiotepa-containing conditioning and autologous stem-cell transplant outlined in the international MARIETTA study demonstrated improvement in outcomes, representing a major accomplishment in the care of patients with DLBCL with SCNSL. Anti-CD19 chimeric antigen receptor T cell denotes a paradigm shift in the treatment of patients with systemic aggressive lymphomas, with emerging data also demonstrating efficacy without higher neurotoxicity in those with SCNSL. In this manuscript we discuss 5 clinical scenarios and review the evidence supporting our recommendations.
Lymphoma that spreads to the central nervous system concurrent with systemic disease (secondary central nervous system [CNS] lymphoma) is a challenging clinical problem with no established standards for treatment. Alderuccio and coauthors used 5 illustrative cases to discuss their approach and recommendations regarding chemoimmunotherapy, autologous stem cell transplantation, and chimeric antigen receptor T-cell therapies.
Introduction
Secondary central nervous system (CNS) involvement by aggressive lymphoma (SCNSL) is an unusual but clinically challenging event historically associated with shorter survival. SCNSL refers to lymphoma spread to the CNS simultaneously with a new diagnosis or during or after treatment of systemic lymphoma. CNS relapse may occur isolated in the CNS or with concurrent systemic involvement.1 The incidence and timing for SCNSL varies based on histology. Diffuse large B-cell lymphoma (DLBCL) accounts for the majority of cases across aggressive histologies, with an incidence of SCNSL of ∼4% to 6%.2, 3, 4 It usually occurs at first relapse within 1 year from diagnosis, with a median time of 5 months (interquartile range [IQR], 2-8).5 In contrast, this event occurs at diagnosis in ∼20% of patients with Burkitt lymphoma (BL).6 SCNSL in mantle-cell lymphoma (MCL) is rare, accounting for 4%, typically occurring at relapse and beyond a year from diagnosis.7,8 The distribution of CNS involvement differs with the histological subtype, with more frequent parenchymal involvement in DLBCL, whereas leptomeningeal infiltration is more common in BL and MCL.4,6,7,9, 10, 11
Clinical6,12, 13, 14, 15, 16 and biological7,17, 18, 19, 20 factors have been associated with a higher risk of SCNSL. The CNS relapse risk in DLBCL is commonly estimated with the CNS International Prognosis Index (IPI) guiding the use of prophylaxis16; however, several recent studies have questioned the efficacy of this practice.4,9,21,22 Recent data suggest that MYC and BCL2 rearrangements only modestly increase the risk of CNS relapse in DLBCL if standard treatments are used.21,23 Two studies implementing multiplatform genomic profiling have refined molecular DLBCL subtypes demonstrating MYD88 L265P, CD79b, ETV6, PIM1, BTG1, and TBL1XR1 mutations in patients assigned to the C5 and MCD subtypes, which suggests the association of these signatures with CNS involvement.30,31 Histological subtype influences decisions to use systemic chemotherapy agents capable of crossing the brain-blood barrier (BBB; eg, high-dose methotrexate [MTX], cytarabine, ifosfamide, or thiotepa), intrathecal (IT) chemotherapy, or novel agents. The risk of SCNSL in BL is substantial with treatment regimens uniformly incorporating CNS-directed therapy.32,33
SCNSL remains a therapeutic challenge commonly characterized by acute neurological symptoms requiring urgent treatment with agents that cross the BBB. Concurrent systemic disease, if/when present, requires rationalization of such therapies. The rarity of this entity as well as the frequent exclusion of patients with SCNSL in clinical trials limits prospective data, delineating optimal management of such patients. In this article, we discuss 5 clinical cases representing the most frequent scenarios of patients with SCNSL by DLBCL, BL, and MCL. Data to support such approaches as well as clinical recommendations are provided for each scenario.
Clinical cases
Clinical case 1: systemic DLBCL with parenchymal CNS involvement at diagnosis
Presentation
A 48-year-old woman presented with left arm weakness and unintentional weight loss associated with neck lymphadenopathy. Positron emission tomography/computed tomography (PET/CT) revealed widespread fluorodeoxyglucose-avid lymphadenopathy associated with liver and bone lesions. Brain magnetic resonance imaging (MRI) with contrast showed an enhancing lesion in the right parietal lobe. Excisional neck and stereotactic brain needle biopsy specimen demonstrated non-germinal center B-cell-like (GCB) DLBCL with BCL6 rearrangement. Her Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 1, and serum lactate dehydrogenase (LDH) level elevated with an IPI of 3.
Discussion
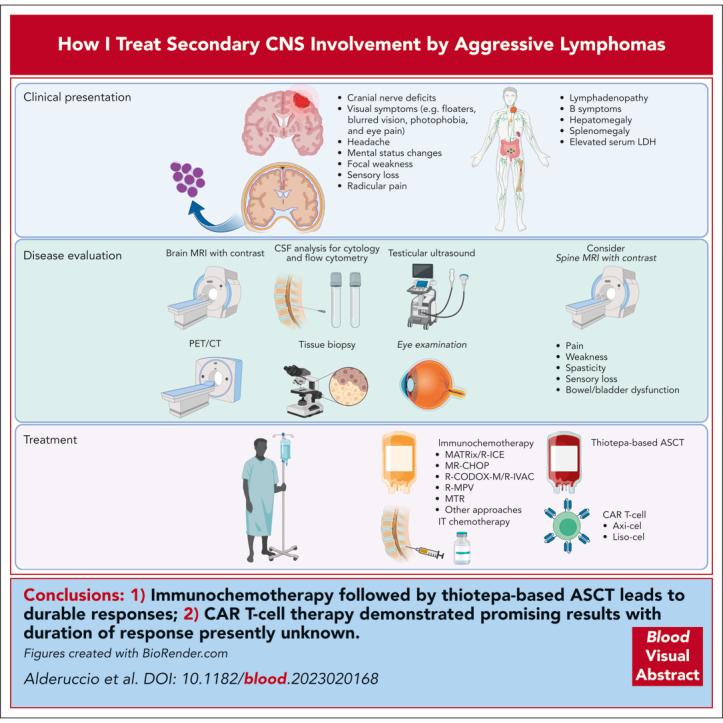
The initial assessment of patients with CNS involvement requires PET/CT associated with neuroimaging, including MRI of the brain with contrast and spinal MRI for those with symptoms. Data supporting PET/CT to appropriately assess testicular involvement in patients with SCNSL is insufficient, and assessment with testicular ultrasound is recommended.1,34 Imaging features of leptomeningeal involvement include focal or diffuse abnormal enhancement of the leptomeninges, cranial nerves, or periventricular region.35 Single or multiple periventricular, uniformly enhancing lesions are characteristic of parenchymal involvement. Lumbar puncture for cytology and flow cytometry analysis of the cerebrospinal fluid (CSF) remain as critical steps in the initial assessment of these patients and are recommended when there is no apparent clinical or contraindication on neuroimaging. Molecular analysis of immunoglobulin heavy chain rearrangement and MYD88 L265P mutation are not essential but may help support the diagnosis.36, 37, 38, 39 Circulating tumor DNA in the CSF and blood is an emerging biomarker in CNS lymphomas, with a potential to better identify, risk-stratify, and monitor response to treatment, although it is not yet readily available for clinical use.40, 41, 42 Initial workup should include confirmatory brain biopsy when feasible, commonly obtained by stereotactic biopsy. For clinical scenarios in which this is not possible, a diagnosis of SCNSL may be made if a systemic biopsy confirms high-grade lymphoma and MRI appearances are consistent with SCNSL, as determined by expert neuro-radiology review. Steroids may reduce or eliminate abnormal contrast enhancement in the MRI and disrupt cellular morphology, resulting in a nondiagnostic pathologic specimen. Thus, steroids should be postponed, if clinically feasible, until diagnostic workup concludes.43 Finally, ophthalmological examination is recommended to determine vitreoretinal involvement.44 Our approach in the diagnostic workup of patients with SCNSL is described in Figure 1.
Figure 1.
Diagnostic evaluation and treatment approach for patients with secondary CNS lymphoma. Secondary CNS involvement in aggressive lymphomas can be leptomeningeal or parenchymal, with symptoms related to disease location. Initial assessment includes neuroimaging and PET/CT associated with CSF evaluation (cytology and flow cytometry) and appropriate tissue biopsies. Testicular ultrasound is also required to complete the assessment for male patients. Treatment options comprise systemic immunochemotherapy with agents capable to cross the BBB and IT chemotherapy associated with ASCT, anti-CD19 CAR T-cell therapy, or BTKis, when appropriate.
Patient-determined factors, such as age, ECOG PS, comorbidities, and overall neurological status influence the choice of frontline therapy. Expeditious control of CNS disease is crucial.45 Hence, patients presenting with acute neurological symptoms may benefit from the use of steroids with high-dose MTX with or without rituximab after a confirmatory biopsy. Frontline DLBCL regimens often include agents such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), which do not cross the BBB; therefore, the incorporation of systemic agents with this capability are required.
The IELSG42 trial incorporating the MARIETTA regimen is the largest clinical trial focused on patients with SCNSL (Table 1).5 This international phase 2 study evaluated an intensive CNS-directed approach composed of 3 cycles of MATRix (rituximab, high-dose MTX, cytarabine, and thiotepa), followed by 3 cycles of R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) and consolidation with carmustine-thiotepa autologous stem-cell transplant (ASCT) for 77 patients with SCNSL (43% at diagnosis). The rationale to include MATRix for patients with SCNSL was based on the impressive results observed in the randomized phase 2 IELSG32 trial for patients with primary CNS lymphoma (PCNSL), for whom this regimen demonstrated better complete response (CR) rates than high-dose MTX and cytarabine with or without rituximab (49% vs 30% vs 23%, respectively).46,47 Superior progression-free survival (PFS) and overall survival (OS) rates were also observed with MATRix. R-ICE is an effective regimen to control systemic lymphoma, with some data also supporting its use in CNS lymphoma.48, 49, 50 After MATRix-R-ICE, the overall response rate (ORR) was 65% with a CR of 39%. The 1-year PFS (study primary end point) was 58% across the entire study population, reaching 100% for those receiving consolidation with ASCT. Toxicity was largely hematologic with grade ≥3 neutropenia (61%), thrombocytopenia (60%), and anemia (35%). The treatment-related mortality of this regimen was 5%, predominantly secondary to sepsis.
Table 1.
Clinical trials in DLBCL with SCNSL
| Study | Regimen | Primary end point | Histology | N | Presentation, % | Median age (range), y | ASCT conditioning | Response, % | Survival, % | TRM, % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. MARIETTA5 | MATRix × 3 R-ICE × 3 Liposomal Ara-C or triple IT |
1-y PFS | DLBCL | 75 | De novo, 43 CNS relapse, 20 CNS-systemic relapse, 37 |
58 (23-70) | Carmustine Thiotepa |
ORR, 61 CR, 55 |
1-y PFS, 58 2-y OS, 46 |
5 |
| 2. SCNSL124 | HD-MTX and Ara-C R-HDS Liposomal Ara-C IT |
2-y EFS | DLBCL, 84% MCL, 8% FL gr 3, 8% |
38 | De novo, 42 CNS relapse, 40 CNS-systemic relapse, 18 |
59 (36-70) | Carmustine Thiotepa |
ORR, 63 CR, 63 |
2-y EFS, 50 5-y OS, 41 |
10 |
| 3. NCT0114817325 | HD-MTX and ifosfamide Ara-C and thiotepa Liposomal Ara-C IT |
TTF | DLBCL, 90% PTCL, 10% |
30 | CNS relapse, 80 CNS-systemic relapse, 20 |
58 (29-65) | Carmustine Thiotepa Etoposide |
ORR, 71 CR, 63 |
2-y TTF, 49 2-y OS, 63 |
3 |
| 4. HOVON 8026 | R-DHAP × 3 HD-MTX × 3 Rituximab IT |
1-y PFS | DLBCL, 97% FL gr 3, 3% |
36 | CNS relapse, 44 CNS-systemic relapse, 56 |
57 (23-65) | Busulfan Cyclophosphamide |
ORR, 53 CR, 22 |
1-y PFS, 19 1-y OS, 25 |
5 |
| 5. UK NCRI27 | R-CODOX-M/IVAC | 2-y PFS | DLBCL | 10 | De novo, 100 | 50∗ (18-65) | — | ORR, 74∗ CR, 47 |
2-y PFS, 70 | 4∗ |
| 6. NCT0231532628 | Ibrutinib, HD-MTX, and rituximab | MTD | DLBCL | 6 | De novo, 50 CNS relapse, 50 |
62∗ (23-74) | - | ORR, 67 CR, 33 |
1-y OS, 71∗ | 0 |
| 7. NCT0396409029 | TEDDI-R | PFS | DLBCL, 55% HGBL, 33% |
49 | De novo, 10 CNS relapse, 90 |
62 (26-89) | - | ORR, 92† CR, 77† |
1-y PFS, 37 1-y OS, 61 |
2 |
Studies 1 to 4 exclusively enrolled patients with SCNSL. Study 5 enrolled 111 patients with untreated DLBCL, including 10 (9%) patients with SCNSL. Study 6 was a phase 1b trial that enrolled 15 patients with PCNSL (n = 9; 60%) and SCNSL (n = 6; 40%).
Ara-C, cytarabine; EFS, event-free survival; FL, follicular lymphoma; gr, grade; HD, high-dose; PTCL, peripheral T-cell lymphoma; R-DHS, rituximab, cyclophosphamide, cytarabine, and etoposide; TEDDI-R, temozolomide, etoposide, liposomal doxorubicin, dexamethasone, ibrutinib, rituximab; TRM, treatment-related mortality; TTF, time-to-treatment failure; MTD, maximum tolerated dose.
Data from the entire study cohort.
In 26 patients with response to ibrutinib.
Caveats of the MATRix-R-ICE-thiotepa-ASCT (MARIETTA) study include the lack of anthracyclines in the MATRix-R-ICE regimen and exclusion of patients aged >70 years and people living with HIV (PLWH). Anthracyclines have been a key component of frontline regimens in aggressive lymphomas, and the absence of this agent is a concern for treatment-naïve patients. However, this regimen allowed for disease debulking with up to 2 cycles of R-CHOP without decreasing the efficacy of this approach (2-year PFS of 89%). Furthermore, the main cause of death in this population is the lack of control of CNS disease; thus, such regimens that address this are recommended. Although the MARIETTA trial excluded PLWH, a retrospective study demonstrated the feasibility of using the MATRix regimen for these patients.51 The same analysis showed poorer tolerability and inferior outcome for patients aged >70 years, highlighting the need for alternative strategies for older patients.
The National Comprehensive Cancer Network guidelines suggest systemic high-dose MTX with or without IT chemotherapy in combination with R-CHOP for patients with concomitant systemic and CNS lymphoma.52 The combination of high-dose MTX with R-CHOP (MR-CHOP) was evaluated in a small clinical trial including 7 patients with PCNSL.53 Patients received high-dose MTX, rituximab, and prednisone on day 1, with successive administration of CHOP backbone on day 3. Subsequently, patients underwent high-dose chemotherapy with ASCT. MR-CHOP demonstrated an ORR of 100% with a CR of 86%. Adverse events were mainly hematologic. Several retrospective studies (n = 21-60) analyzed outcomes with MR-CHOP–like regimens in SCNSL, reporting an ORR range from 66% to 80% and a CR from 57% to 68%.54, 55, 56, 57, 58 Consolidation with ASCT was commonly associated with an improved 3-year PFS (75% vs 26%; P = .001) and OS (75% vs 29%; P = .002).54 Presence of active CNS lymphoma before ASCT has been consistently associated with worse outcomes54,59,60; however, the use of thiotepa-based regimens demonstrated similar outcomes between patients achieving partial responses (CNS or systemic) and those in CR before ASCT.61
Data from 10 patients with exclusively leptomeningeal SCNSL evaluating the dose-dense R-CODOX-M (rituximab, cyclophosphamide, vincristine, doxorubicin, and high-dose MTX)/R-IVAC (rituximab, ifosfamide, etoposide, and high-dose cytarabine) regimen for patients with high IPI DLBCL demonstrated a 2-year PFS of 70%.27 Most common nonhematologic grade ≥3 toxicities included infections (71%), mucositis (32%), and febrile neutropenia (18%). Importantly, patients with an ECOG PS of 3 and age ≥50 years experienced higher early treatment discontinuation, including 5 treatment-related deaths, underscoring the need for caution in this population. Furthermore, high-dose MTX was delivered over 24 hours in the original protocol. The regimen has been revised at many centers, administering MTX over a 3-hour period because better BBB penetration has been demonstrated and is regularly recommended in PCNSL protocols.47,62,63 Contrary to MARIETTA and MR-CHOP regimens in which thiotepa-based ASCT consolidation is a key component, the role of this approach after R-CODOX-M/R-IVAC has not been evaluated. Finally, several studies have confirmed the feasibility of intensive regimens for PLWH.51,64,65 Hence, effective treatment strategies should always be considered for those receiving appropriate combination antiretroviral therapy.
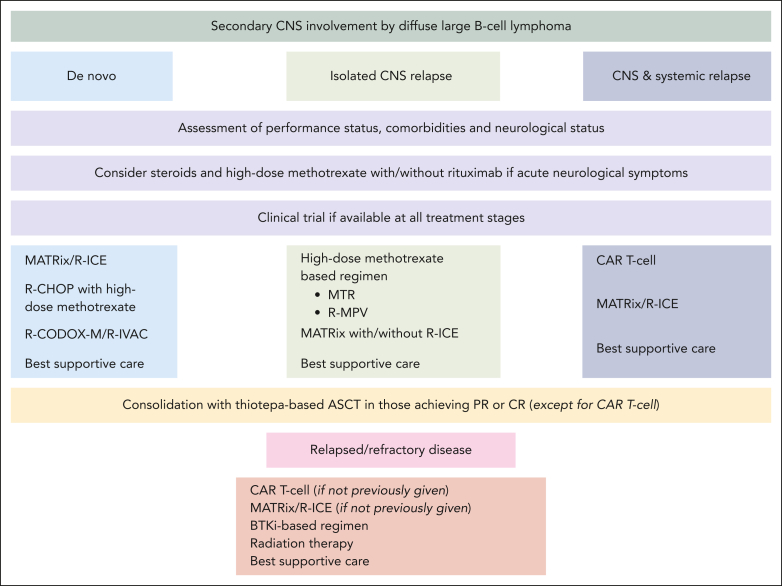
Guided decision-making
MARIETTA regimen and MR-CHOP represent appropriate options for patients aged <70 years. For those treated with MR-CHOP who have adequate renal function (creatinine clearance [CrCl] ≥ 40 mL/min),66 we recommend at least 3 g/m2 of systemic MTX delivered over 3 hours,67,68 followed by thiotepa-based ASCT consolidation for responders (Figure 2). MR-CHOP also emerges as an option and consideration of consolidation, with dose-modified thiotepa–conditioned ASCT for older patients.69 However, close monitoring for toxicity, dose adjustment based on CrCl before each cycle, and possible drug omissions may be required for this population.
Figure 2.
Treatment approach for 3 possible clinical scenarios of secondary CNS involvement by DLBCL. PR, partial response.
Recommendation
The patient received 1 cycle of debulking R-CHOP, followed by 3 cycles of MATRix and R-ICE. After developing neutropenic fever with the first course of MATRix, 1 dose of cytarabine (fourth dose) was omitted for subsequent cycles. The patient achieved systemic and CNS CR, proceeding with thiotepa-containing conditioning and ASCT, achieving an ongoing remission 26 months after diagnosis.
Clinical case 2: DLBCL with CNS relapse without systemic disease
Presentation
A 52-year-old woman presented with a pathological fracture of the left ankle, and a surgical bone biopsy helped confirm GC-DLBCL. Staging PET/CT demonstrated fluorodeoxyglucose-avid paranasal, liver, and left ankle lesions associated with pelvic lymphadenopathy. Brain MRI with contrast and CSF assessment showed normal results. Serum LDH level was elevated, with an ECOG PS of 2 and IPI and CNS-IPI scores of 4. She achieved CR on PET/CT after 6 cycles of R-CHOP and CNS prophylaxis with IT chemotherapy and 2 cycles of high-dose MTX. Three months after finishing therapy, the patient developed acute-onset left sided weakness. Brain MRI showed an enhancing lesion in the right basal ganglia, with stereotactic biopsy confirming CNS DLBCL relapse, without systemic lesion on PET/CT.
Discussion
CNS recurrence in DLBCL usually occurs during or within a few months of completing frontline therapy, suggesting subclinical disease at diagnosis.10 The addition of rituximab to frontline regimens appears to have reduced the risk of CNS relapse.3,70, 71, 72, 73 MTX-based therapies, including R-MPV (rituximab, MTX, procarbazine, and vincristine), MTR (MTX, temozolomide, and rituximab), and MATRix have demonstrated high efficacy in PCNSL, with CR rates >60%.74,75 Furthermore, the use of these regimens followed by ASCT as consolidation of frontline therapy or in the relapse setting is associated with durable remissions.76, 77, 78, 79, 80, 81, 82 Similar results were also observed in patients with CNS relapse of aggressive lymphomas.61,83 Korfel et al reported a 2-year time-to-treatment failure of 58%, with the median OS not reached, after induction chemotherapy followed by carmustine-thiotepa-ASCT (n = 30).25 Grade ≥3 toxicity was largely hematologic, with 1 death occurring during treatment. The MARIETTA trial enrolled patients with isolated CNS relapse, reporting better survival than those with systemic and CNS relapse, as described recently.61 Although the outcome in the MARIETTA trial for this group of patients was inferior to those with CNS-systemic disease at initial diagnosis, the numbers were small (n = 15 [20%]).5
CD19 chimeric antigen receptor (CAR) T cell represents a paradigm shift in the treatment of aggressive lymphomas; however, because of concerns about potential severe immune effector cell–associated neurotoxicity syndrome (ICANS), patients with SCNSL were excluded from the ZUMA-1 and JULIET studies.84,85 Subsequent small series (n = 5-9) reported CR rates from 55% to 60%, with a single case of grade 4 ICANS after axicabtagene ciloleucel (axi-cel).86,87 Studies in acute lymphoblastic leukemia demonstrated CAR T-cell trafficking, expansion, and persistence into the CSF supporting the use of this approach in SCNSL.88, 89, 90 Consequently, Frigault et al investigated tisagenlecleucel (tisa-cel) in 12 patients with relapsed/refractory (r/r) PCNSL.91 All required bridging therapy to address symptomatic disease after leukapheresis. Grade 1 cytokine release syndrome (CRS) occurred in 7 (58%) and ICANS in 6 (50%) patients, with a single case of grade 3 associated with peak CAR T-cell expansion. Encouraging efficacy was observed in this study, with an ORR of 58% and a CR of 50%. CAR T-cell advancement into the CSF was observed in all patients and higher levels of CAR transgene RNA in the CSF of those achieving CR. From a retrospective series in SCNSL, responses appear better with axi-cel (ORR of 86% with all responders achieving CR) than with tisa-cel (ORR of 50% with a CR rate of 25%), although neurotoxicity (grade 3 ICANS, 37.5%) rates were higher.92,93
Genomic interrogation of patients with PCNSL determined frequent 9p24.1 alterations, attracting interest in checkpoint inhibitors.94 Single-agent nivolumab showed encouraging efficacy in a small cohort of patients with r/r PCNSL and primary testicular lymphoma with CNS relapse, but this was not replicated in a multicenter trial.95,96 Furthermore, checkpoint inhibitors have failed to demonstrate significant activity in systemic DLBCL, questioning its use in SCNSL.97,98 Other novel agents have also been evaluated for this population. Lenalidomide in combination with rituximab demonstrated a CR rate of 33% among 6 patients with SCNSL.99 Similar results were observed in PCNSL (n = 34), with a CR rate of 35% but a median PFS of only 3.9 months, underscoring the limited efficacy of this combination.100 The Bruton tyrosine kinase inhibitors (BTKis) ibrutinib and zanubrutinib have demonstrated encouraging preliminary activity in PCNSL and SCNSL, with responses >67%; however, durability with single-agent ibrutinib was also limited (<6 months).28,101, 102, 103 In a response-adapted approach, temozolomide, etoposide, liposomal doxorubicin, dexamethasone, ibrutinib, and rituximab demonstrated encouraging results in SCNSL, particularly for those with CD10– tumors (1-year PFS 50.5% vs 15.4%; P = .03).29
Clinical decision-making
For patients with isolated CNS relapse of DLBCL, the MATRix regimen (with/without R-ICE), MTR, and R-MPV have demonstrated excellent results and should be considered standard approaches. Rituximab with high-dose MTX combination therapy is an appropriate alternative, especially for older patients. Consistent with our recommendation in case 1, we favor the use of MTX with a dose >3 g/m2 for older patients with normal CrCl.81 Those patients with responsive disease should be considered for consolidation with thiotepa-ASCT, if appropriate. For patients with residual CNS disease after ASCT or disease progression during treatment, whole-brain radiation therapy may be considered because it is a valuable option with the potential to improve outcomes. Further data are advocated for CAR T-cell therapy and BTKi/lenalidomide-containing approaches.
Recommendation
The patient received 8 cycles of MTR without related toxicity. She achieved CR, proceeding with thiotepa-containing conditioning and ASCT, remaining in ongoing remission for 3 years.
Clinical case 3: relapsed DLBCL with concomitant systemic and CNS disease after frontline therapy
Presentation
A 59-year-old man with prior history of stage II non-GCB DLBCL treated with 6 cycles of R-CHOP presented with new abdominal lymphadenopathy associated with headache and left visual field deficit 4 months after the end of therapy. Brain MRI demonstrated a right parieto-occipital enhancing lesion, with stereotactic biopsy confirming DLBCL relapse.
Discussion
Patients with early concomitant relapse of SCNSL remain a therapeutic challenge, with worse outcomes compared with those with isolated CNS relapse.61 In this setting, CNS involvement is more commonly characterized by leptomeningeal involvement, and CNS progression during frontline therapy is associated with even shorter survival.104 Patients with concomitant CNS and systemic relapse in MARIETTA study achieved lower response (ORR = 43% with a CR rate of 32%) and survival rates (2 year PFS, 14%) than those in other subgroups.5
Contrary to the ZUMA-1 and JULIET studies,84,85 the TRANSCEND trial treated 7 patients with SCNSL, demonstrating the feasibility of CAR T-cell in this scenario (Table 2).105 The randomized TRANSFORM trial allowed for the enrollment of patients with SCNSL; however, only 1 patient was enrolled in the lisocabtagene maraleucel (liso-cel) cohort.110 For patients with SCNSL, with and without concomitant systemic disease, CAR T-cell therapy does not seem to be associated with increased risk of neurotoxicity.108,111 Across all CAR T-cell products, patients with SCNSL demonstrated an incidence of CRS of 72% (grade ≥3, 11%) and ICANS of 48% (grade ≥3, 26%). In the registrational trials, axi-cel recorded higher rates of CRS and ICANS than tisa-cel and liso-cel.111 Furthermore, bridging strategies can be challenging and may include radiation therapy techniques, and evaluation of long-term neurocognitive outcomes of this approach are awaited.107 In a postapproval analysis, patients with and without SCNSL treated with axi-cel achieved similar response (CR at 12 months, 50% vs 65%; P = .210) and survival rates (12-month PFS rate, 44% vs 47%, respectively; P = .230).106,112 Furthermore, CAR T-cell therapy appears efficacious in both parenchymal and leptomeningeal disease.108 Limited data support the use of allogeneic-SCT in SCNSL, although a retrospective analysis (n = 20) reported a median PFS of 3.8 years but was associated with a 4-year nonrelapse mortality rate of 30%.113
Table 2.
Selected studies evaluating the safety and efficacy of CAR T-cell therapy in SCNSL
| Study | Product | N | Response, % | Survival, mo | CRS, % | Neurotoxicity, % |
|---|---|---|---|---|---|---|
| TRANSCEND105 | Liso-cel | 7∗ | ORR, 50 CR, 50 |
NR | Any grade, 29 Grade ≥3, 0 |
Any grade, 29 Grade ≥3, 29 |
| NCT0460848793 | Axi-cel | 9† | ORR, 78 CR, 67 |
mPFS, 11.5‡ | Any grade, 88 Grade ≥3, 0 |
Any grade, 50 Grade ≥3, 37.5 |
| Retrospective study106 | Axi-cel | 17§ | ORR, 59 CR, NR |
6-month EFS from infusion, 50% | Any grade, 93 Grade ≥3, 13 |
Any grade, 87 Grade ≥3, 33 |
| Retrospective study92 | Tisa-cel | 8 | ORR, 50 CR, 25 |
NR | Any grade, 87 Grade ≥3, 0 |
Any grade, 37 Grade ≥3, 0 |
| Retrospective study107 | Tisa-cel, 57% Axi-cel, 43% |
7 | ORR, 86 CR, 86 |
mPFS, 2.8 mOS, 4.3 |
Any grade, 57 Grade ≥3, 14 |
Any grade, 43 Grade ≥3, 14 |
| Retrospective study108 | Axi-cel | 5 | ORR, 80‖ CR, 60 |
mPFS, 4.5 mOS, 5.2 |
Any grade, 40 Grade ≥3, 0 |
Any grade, 40 Grade ≥3, 40 |
| Retrospective study109 | Costimulatory domain 4-1BB, 80% CD28, 20% |
10 | ORR, 70¶ CR, 20 |
mPFS (systemic), 3 mPFS (CNS), not reached |
Any grade, 100 Grade ≥3, 10 |
Any grade, 60 Grade ≥3, 30 |
EFS, event-free survival; mPFS, median PFS; mOS, median OS; NR, not reported.
Six patients evaluable for response.
PCNSL, 67% and SCNSL, 33%.
Ongoing accrual and follow-up.
Fifteen patients evaluable for response.
One patient with stable disease.
Three patients with stable disease.
For patients with concomitant systemic and CNS lymphoma relapse, CAR T-cell is emerging as an approach capable of overcoming the dismal historically reported outcomes. Axi-cel and liso-cel are presently approved for refractory DLBCL or relapse within 12 months of frontline therapy, although patients with SCNSL were either excluded or poorly represented in the pivotal randomized trials ZUMA-7 and TRANSFORM.
Clinical decision-making
Immunochemotherapy regimens have not been associated with significant survival benefit, yet CAR T-cell therapy appears effective in this scenario. For the treatment of concomitant systemic and CNS relapse, we recommend CAR T-cell therapy, if accessible. In cases in which CAR T-cell is not an option, we favor the use of MARIETTA regimen.
Recommendation
High-dose MTX (3.5 g/m2) and cytarabine were initiated as a bridge to CAR T-cell therapy. He subsequently received axi-cel with CRS grade 2 and ICANS grade 1. PET/CT on day +30 demonstrated CR and no evidence of disease on brain MRI or CSF. The patient remains in ongoing systemic and CNS remission 6 months after CAR T-cell therapy.
Clinical case 4: BL associated with CNS involvement at diagnosis
Presentation
A 36-year-old man was evaluated for severe back pain and hypoesthesia of the chin compatible with mental nerve infiltration. Workup demonstrated widespread lymphadenopathy and hepatosplenomegaly associated with serum LDH level >3 times upper limit of normal. Lymph node and bone marrow biopsies demonstrated involvement by BL, and CSF analysis confirmed leptomeningeal involvement. The patient was diagnosed with HIV infection with a CD4 count of 400/μL and initiated combination antiretroviral therapy. His ECOG PS was 1 (BL-IPI score = 2).
Discussion
BL is a malignancy associated with a substantial risk of SCNSL and affinity for leptomeninges. At diagnosis, ∼20% to 30% of the patients demonstrate CNS disease.6,114 Historical literature reports a risk of CNS relapse of up to 50% without CNS prophylaxis.115,116 Therefore, contemporary immunochemotherapy regimens regularly incorporate CNS prophylaxis, decreasing this risk to ∼6%.6 SCNSL is a high-risk factor in BL despite the use of modern regimens and universally occurs within the first year from diagnosis.6,114,117 Risk factors associated with SCNSL are HIV infection, elevated LDH level, poor ECOG PS, ≥2 extranodal sites, and bone marrow involvement.2,6 Presence of SCNSL represents an independent prognostic factor for shorter survival, without significant differences between parenchymal and leptomeningeal disease. Common factors associated with CNS relapse after frontline therapy are advanced-stage disease and prior CNS involvement.
The treatment landscape of BL can be classified into high-intensity immunochemotherapy regimens with systemic CNS-penetrating agents and IT chemotherapy, such as R-CODOX-M/R-IVAC and hyper-CVAD-R (hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone with rituximab alternating with high-dose MTX and cytarabine), and the low-intensity regimen dose-adjusted EPOCH-R (Table 3). The latter provides CNS prophylaxis by IT MTX, with both intensity approaches recommending an enhanced CNS-directed therapy in the presence of SCNSL.32,122 Prospective single-arm studies have demonstrated the ability of high-intensity immunochemotherapy regimens to appropriately address SCNSL.118,119,122 The incidence of CNS relapse with dose-adjusted EPOCH-R in clinical trials is 2%.32,120 However, patients with parenchymal CNS disease were not enrolled into clinical trials evaluating this approach, and those with baseline leptomeningeal disease experienced significantly shorter 4-year event-free survival (45.5% vs 90%; P = .0004).
Table 3.
Clinical trials for BL with SCNSL
| Study | Regimen | N | CNS involvement, % | Survival for all patients, % | Survival for patients with CNS involvement | TRM of all patients, % | CNS relapse, % |
|---|---|---|---|---|---|---|---|
| UKLG LY06118 | CODOX-M IVAC |
52 | 13 | 2-y EFS, 65 2-y OS, 73 |
1-y, 100 | 6 | NR |
| AMC048119 | R-CODOX-M R-IVAC |
34 | 12 | 1-y PFS, 69 2-y OS, 69 |
NR | 3 | 3 |
| NCT00001337120 | DA-EPOCH-R | 19 | 5 | FFP, 95 OS, 100 |
OS, 100 | 0 | 0 |
| NCT0109218232 | DA-EPOCH-R | 113 | 10 | 4-y EFS, 84 4-y OS, 87 |
4-y EFS, 45 | 4 | 3 |
| MDACC single-arm study121 | HyperCVAD-R | 31∗ | 6 | 3-y EFS, 80 3-y OS, 89 |
3-y OS, 50 | NR | 0 |
| Retrospective study114 | HyperCVAD-R | 54 | 28 | 5-y RFS, 59 5-y OS, 55 |
5-y RFS, 26 5-y OS, 23 |
15 | 4 |
DA-EPOCH-R, dose-adjusted infusional EPOCH-R; EFS, event-free survival; FFP, freedom from progression; MDACC, MD Anderson Cancer Center; NR, not reported; RFS, relapse-free survival; TRM, treatment-related mortality.
BL, n = 17 (55%) and B-cell acute lymphoblastic leukemia, n = 14 (45%).
The use of dose-adjusted EPOCH-R compared with high-intensity regimens is an attractive strategy aiming to decrease toxicity. The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) study compared R-CODOX-M/R-IVAC with dose-adjusted EPOCH-R, and although the study closed early, they have reported similar 2-year OS rates but higher toxicity in the R-CODOX-M/R-IVAC arm.123 This study also excluded patients with baseline CNS involvement; therefore, prospective studies have not addressed whether dose-adjusted EPOCH-R addresses SCNSL similar to high-intensity regimens. Furthermore, in a large retrospective study, dose-adjusted EPOCH-R was associated with higher risk (13%; subhazard ratio = 3.6) of CNS relapse than high-intensity regimens (4%; P < .001).6
Achievement of CR in BL is strongly associated with survival, and patients with r/r disease have a dismal prognosis independent of salvage approach, and no standard second-line regimen exists.64,124,125 Emerging data demonstrated encouraging efficacy of CAR T-cell therapy for pediatric patients with SCNSL, demonstrating an 18-month PFS of 60%.126 The ZUMA-25 study (NCT05537766) is currently enrolling patients with r/r BL and will provide data on the efficacy of CAR T-cell in this setting; however, this study excludes patients with SCNSL.
Clinical decision-making
We suggest the use of high-intensity immunochemotherapy regimens with systemic CNS-penetrating agents for patients with SCNSL in BL. We favor R-CODOX-M/R-IVAC over hyper-CVAD-R based on retrospective data reporting lower treatment-related mortality.124
Recommendation
The patient initiated immunochemotherapy with R-CODOX-M/R-IVAC and appropriate IT chemotherapy schedule. He is in an ongoing remission for 24 months.
Clinical case 5: MCL with isolated CNS relapse
Presentation
A 52-year-old man with a diagnosis of stage IV MCL characterized by bone marrow involvement, widespread lymphadenopathy, and associated weight loss received alternating R-CHOP with rituximab, dexamethasone, cytarabine, and cisplatin (R-DHAP) followed by ASCT and rituximab maintenance. The patient relapsed 3 years later with cervical lymphadenopathy, confirmed upon biopsy, and without evidence of TP53 mutation. He initiated second-line treatment with bendamustine and rituximab. He remained in remission for 11 months, when he developed an intractable headache associated with unstable gait. Brain MRI demonstrated leptomeningeal enhancement, with CSF flow cytometry–positive results for neoplastic B-cell population compatible with MCL.
Discussion
SCNSL in MCL is a rare event commonly occurring at relapse with predominant leptomeningeal involvement. Factors associated with higher risk for SCNSL are blastoid histology, advanced-stage, B-symptoms, elevated LDH level, ECOG PS ≥ 2, high MCL International Prognostic Index (MIPI) score, and Ki67 ≥ 30.7,17,127 No standard therapy exists for MCL with SCNSL, but as observed in systemic MCL, ibrutinib also demonstrates activity in CNS disease.128,129 In a retrospective study including 88 patients, ibrutinib at 560 mg daily was associated with better response (ORR 78% with a CR rate of 46% vs ORR 46% with a CR rate of 21%; P = .031) and survival rates (median PFS, 13.1 vs 3 months; P = .009) than those with BBB-penetrating chemotherapy.8 The addition of IT chemotherapy did not translate into better survival, irrespective of treatment approach. Despite the success of ibrutinib, multiple mechanisms of resistance have been described, including the emergence of C481S-BTK mutation. Data from noncovalent BTKis are awaited in this setting. Selective BTK degraders represent a novel approach to overcome emergent resistance mutations. These compounds possess the capability to degrade wild-type and C481S-BTK mutation in preclinical models, and some agents are capable of penetrating the BBB, with activity described in CNS lymphomas.130,131 Clinical trials testing selective BTK degraders in CNS lymphomas are ongoing, and these compounds may represent an option for patients progressing with ibrutinib in the future. In MCL, similar to DLBCL, the ZUMA-2 trial led to the approval of brexucabtagene autoleucel, but patients with SCNSL were excluded.132 The role of CAR T-cell therapy in this setting remains unclear; however, a postapproval study demonstrated a CR rate of 75% without shorter PFS or higher grade ≥3 neurotoxicity in 16 patients with SCNSL.133
Clinical decision-making
For patients with SCNSL by MCL, we favor the use of ibrutinib over immunochemotherapy regimens. For those progressing with ibrutinib, enrollment to BTK degraders or CAR T-cell clinical trials emerge as an attractive option.
Outcome
The patient initiated ibrutinib 560 mg daily, remaining in ongoing remission for 8 months.
Conclusions
SCNSL is a serious event associated with shorter survival regardless of histology. For those with concomitant systemic and CNS DLBCL relapse, the prognosis remains especially unsatisfactory. The treatment of patients with SCNSL has improved considerably with the development of more effective therapies, such as the MARIETTA regimen, demonstrating improved outcomes for patients with secondary CNS at diagnosis and relapse. CAR T-cell therapy represents a major advance in the treatment of r/r aggressive lymphomas with recent data also demonstrating efficacy in treating CNS lymphoma; however, durability of response remains to be established. Furthermore, immunochemotherapy followed by thiotepa-based ASCT demonstrated durable remissions, highlighting the need for randomized data exhibiting a survival benefit before CAR T-cell therapy becomes the new standard-of-care. Regimens incorporating systemic agents capable of penetrating the BBB better serve patients with BL presenting with SCNSL. However, outcomes remain unsatisfactory representing an unmet need in the field. In contrast, the efficacy of immunochemotherapy appears limited for patients with SCNSL involvement by MCL, with improved outcomes with BTKis. Currently, there are no prospective data supporting the use of CD3 × CD20 bispecific antibodies in SCNSL.134, 135, 136 Future clinical trials assessing novel agents in aggressive lymphomas should regularly include those with SCNSL to expand the treatment landscape for a population with limited treatment options with the aim of improving outcomes.
Conflict-of-interest disclosure: J.P.A. provided consultancy for ADC Therapeutics, AbbVie, and Genentech; received research funding from ADC Therapeutics; and had an immediate family member serve on the advisory boards of Servier and Forma Therapeutics. L.N. has consulted for Ono Pharmaceutical, Brave Bio, Genmab, and Kite/Gilead; has received clinical trial research support from Bristol Myers Squibb, Ono, Merck, Kazia, and AstraZeneca; and delivered continuing medical education lectures for Oakstone Medical Publishing and Neurology Audio Digest. K.C. has played a consulting/advisory role for Roche, Takeda, Celgene, Atara, Gilead, Kite, Janssen, AbbVie, and Incyte; served on speakers bureaus for Roche, Takeda, Kite, Gilead, and Incyte; and received conference/travel support from Roche, Takeda, Kite, Janssen, and Bristol Myers Squibb.
Acknowledgments
Figures 1 and 2 were created with BioRender.com.
J.P.A. is supported by the Sylvester Comprehensive Cancer Center National Cancer Institute core grant (P30CA240139), Peykoff Initiative from the Lymphoma Research Foundation, the Dwoskin Family Foundation, and the US Department of Defense (grant CA220385). L.N. is supported by a Leukemia and Lymphoma Society Career Development Program grant (2322-19).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or US Department of Defense.
Authorship
Contribution: J.P.A., L.N., and K.C. wrote, edited, and approved the manuscript.
Footnotes
∗All authors contributed equally to this work.
References
- 1.Cwynarski K, Cummin T, Osborne W, et al. Management of secondary central nervous system lymphoma. Br J Haematol. 2023;200(2):160–169. doi: 10.1111/bjh.18539. [DOI] [PubMed] [Google Scholar]
- 2.Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18(1):149–157. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- 3.Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111(3):1085–1093. doi: 10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- 4.Wilson MR, Eyre TA, Kirkwood AA, et al. Timing of high-dose methotrexate CNS prophylaxis in DLBCL: a multicenter international analysis of 1384 patients. Blood. 2022;139(16):2499–2511. doi: 10.1182/blood.2021014506. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJM, Doorduijn JK, Re A, et al. MATRix-RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): an international, single-arm, phase 2 trial. Lancet Haematol. 2021;8(2):e110–e121. doi: 10.1016/S2352-3026(20)30366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zayac AS, Evens AM, Danilov A, et al. Outcomes of Burkitt lymphoma with central nervous system involvement: evidence from a large multicenter cohort study. Haematologica. 2021;106(7):1932–1942. doi: 10.3324/haematol.2020.270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheah CY, George A, Giné E, et al. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol. 2013;24(8):2119–2123. doi: 10.1093/annonc/mdt139. [DOI] [PubMed] [Google Scholar]
- 8.Rusconi C, Cheah CY, Eyre TA, et al. Ibrutinib improves survival compared with chemotherapy in mantle cell lymphoma with central nervous system relapse. Blood. 2022;140(17):1907–1916. doi: 10.1182/blood.2022015560. [DOI] [PubMed] [Google Scholar]
- 9.Bobillo S, Joffe E, Sermer D, et al. Prophylaxis with intrathecal or high-dose methotrexate in diffuse large B-cell lymphoma and high risk of CNS relapse. Blood Cancer J. 2021;11(6):113. doi: 10.1038/s41408-021-00506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qualls D, Abramson JS. Advances in risk assessment and prophylaxis for central nervous system relapse in diffuse large B-cell lymphoma. Haematologica. 2019;104(1):25–34. doi: 10.3324/haematol.2018.195834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobillo S, Khwaja J, Ferreri AJM, Cwynarski K. Prevention and management of secondary central nervous system lymphoma. Haematologica. 2023;108(3):673–689. doi: 10.3324/haematol.2022.281457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Galaly TC, Villa D, Michaelsen TY, et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: an international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer. 2017;75:195–203. doi: 10.1016/j.ejca.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 13.El-Galaly TC, Cheah CY, Hutchings M, et al. Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol. 2016;175(5):876–883. doi: 10.1111/bjh.14325. [DOI] [PubMed] [Google Scholar]
- 14.Hosein PJ, Maragulia JC, Salzberg MP, et al. A multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab era. Br J Haematol. 2014;165(3):358–363. doi: 10.1111/bjh.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kridel R, Telio D, Villa D, et al. Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol. 2017;176(2):210–221. doi: 10.1111/bjh.14392. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34(26):3150–3156. doi: 10.1200/JCO.2015.65.6520. [DOI] [PubMed] [Google Scholar]
- 17.Chihara D, Asano N, Ohmachi K, et al. Ki-67 is a strong predictor of central nervous system relapse in patients with mantle cell lymphoma (MCL) Ann Oncol. 2015;26(5):966–973. doi: 10.1093/annonc/mdv074. [DOI] [PubMed] [Google Scholar]
- 18.Conconi A, Franceschetti S, Lobetti-Bodoni C, et al. Risk factors of central nervous system relapse in mantle cell lymphoma. Leuk Lymphoma. 2013;54(9):1908–1914. doi: 10.3109/10428194.2013.767454. [DOI] [PubMed] [Google Scholar]
- 19.Ollila TA, Kurt H, Waroich J, et al. Genomic subtypes may predict the risk of central nervous system recurrence in diffuse large B-cell lymphoma. Blood. 2021;137(8):1120–1124. doi: 10.1182/blood.2020007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood. 2019;133(9):919–926. doi: 10.1182/blood-2018-07-862862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orellana-Noia VM, Reed DR, McCook AA, et al. Single-route CNS prophylaxis for aggressive non-Hodgkin lymphomas: real-world outcomes from 21 US academic institutions. Blood. 2022;139(3):413–423. doi: 10.1182/blood.2021012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre TA, Kirkwood AA, Wolf J, et al. Stand-alone intrathecal central nervous system (CNS) prophylaxis provide unclear benefit in reducing CNS relapse risk in elderly DLBCL patients treated with R-CHOP and is associated increased infection-related toxicity. Br J Haematol. 2019;187(2):185–194. doi: 10.1111/bjh.16070. [DOI] [PubMed] [Google Scholar]
- 23.Alduaij W, Jiang A, Villa D, et al. Risk of central nervous system involvement in high-grade B-cell lymphoma with MYC and BCL2 rearrangements: analysis of a population-based cohort with routine fluorescence in situ hybridization testing in British Columbia. Blood. 2022;140(suppl 1):1332–1333. [Google Scholar]
- 24.Ferreri AJ, Donadoni G, Cabras MG, et al. High doses of antimetabolites followed by high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation in patients with systemic B-cell lymphoma and secondary CNS involvement: final results of a multicenter phase II trial. J Clin Oncol. 2015;33(33):3903–3910. doi: 10.1200/JCO.2015.61.1236. [DOI] [PubMed] [Google Scholar]
- 25.Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica. 2013;98(3):364–370. doi: 10.3324/haematol.2012.077917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doorduijn JK, van Imhoff GW, van der Holt B, et al. Treatment of secondary central nervous system lymphoma with intrathecal rituximab, high-dose methotrexate, and R-DHAP followed by autologous stem cell transplantation: results of the HOVON 80 phase 2 study. Hematol Oncol. 2017;35(4):497–503. doi: 10.1002/hon.2342. [DOI] [PubMed] [Google Scholar]
- 27.McMillan AK, Phillips EH, Kirkwood AA, et al. Favourable outcomes for high-risk diffuse large B-cell lymphoma (IPI 3-5) treated with front-line R-CODOX-M/R-IVAC chemotherapy: results of a phase 2 UK NCRI trial. Ann Oncol. 2020;31(9):1251–1259. doi: 10.1016/j.annonc.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133(5):436–445. doi: 10.1182/blood-2018-09-875732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roschewski M, Simard J, Melani C, et al. Phase 2 of ibrutinib with temozolomide, etoposide, liposomal doxorubicin, dexamethasone, rituximab (TEDDI-R) for secondary CNS lymphoma. Hematol Oncol. 2023;41(S2):44–46. [Google Scholar]
- 30.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679–690. doi: 10.1038/s41591-018-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Burkitt lymphoma. J Clin Oncol. 2020;38(22):2519–2529. doi: 10.1200/JCO.20.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45(4):761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 34.Higgins A, Kim H, Harper L, et al. Testicular FDG-PET/CT uptake threshold in aggressive lymphomas. Am J Hematol. 2021;96(3):E81–e83. doi: 10.1002/ajh.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311–319. doi: 10.1001/jamaneurol.2013.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromberg JE, Breems DA, Kraan J, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68(20):1674–1679. doi: 10.1212/01.wnl.0000261909.28915.83. [DOI] [PubMed] [Google Scholar]
- 37.Ekstein D, Ben-Yehuda D, Slyusarevsky E, Lossos A, Linetsky E, Siegal T. CSF analysis of IgH gene rearrangement in CNS lymphoma: relationship to the disease course. J Neurol Sci. 2006;247(1):39–46. doi: 10.1016/j.jns.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Wilson WH, Bromberg JE, Stetler-Stevenson M, et al. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2014;99(7):1228–1235. doi: 10.3324/haematol.2013.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe J, Natsumeda M, Okada M, et al. High detection rate of MYD88 mutations in cerebrospinal fluid from patients with CNS lymphomas. JCO Precis Oncol. 2019;3(3):1–13. doi: 10.1200/PO.18.00308. [DOI] [PubMed] [Google Scholar]
- 40.Olszewski AJ, Chorzalska AD, Petersen M, et al. Detection of clonotypic DNA in the cerebrospinal fluid as a marker of central nervous system invasion in lymphoma. Blood Adv. 2021;5(24):5525–5535. doi: 10.1182/bloodadvances.2021004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobillo S, Crespo M, Escudero L, et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica. 2021;106(2):513–521. doi: 10.3324/haematol.2019.241208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutter JA, Alig SK, Esfahani MS, et al. Circulating tumor DNA profiling for detection, risk stratification, and classification of brain lymphomas. J Clin Oncol. 2023;41(9):1684–1694. doi: 10.1200/JCO.22.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24(8):1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 44.de la Fuente MI, Alderuccio JP, Reis IM, et al. Bilateral radiation therapy followed by methotrexate-based chemotherapy for primary vitreoretinal lymphoma. Am J Hematol. 2019;94(4):455–460. doi: 10.1002/ajh.25414. [DOI] [PubMed] [Google Scholar]
- 45.Wight JC, Yue M, Keane C, et al. Outcomes of synchronous systemic and central nervous system (CNS) involvement of diffuse large B-cell lymphoma are dictated by the CNS disease: a collaborative study of the Australasian Lymphoma Alliance. Br J Haematol. 2019;187(2):174–184. doi: 10.1111/bjh.16064. [DOI] [PubMed] [Google Scholar]
- 46.Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–227. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- 47.Ferreri AJM, Cwynarski K, Pulczynski E, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. 2022;36(7):1870–1878. doi: 10.1038/s41375-022-01582-5. [DOI] [PubMed] [Google Scholar]
- 48.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moskowitz CH, Schöder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(11):1896–1903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mappa S, Marturano E, Licata G, et al. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol. 2013;31(3):143–150. doi: 10.1002/hon.2037. [DOI] [PubMed] [Google Scholar]
- 51.Schorb E, Fox CP, Kasenda B, et al. Induction therapy with the MATRix regimen in patients with newly diagnosed primary diffuse large B-cell lymphoma of the central nervous system - an international study of feasibility and efficacy in routine clinical practice. Br J Haematol. 2020;189(5):879–887. doi: 10.1111/bjh.16451. [DOI] [PubMed] [Google Scholar]
- 52.NCCN . 2023. Clinical Practice Guidelines in Oncology. B-Cell Lymphomas vAJ, 2023.https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf [Google Scholar]
- 53.Masaki Y, Miki M, Sun Y, et al. High-dose methotrexate with R-CHOP therapy for the treatment of patients with primary central nervous system lymphoma. Int J Hematol. 2011;93(6):720–726. doi: 10.1007/s12185-011-0848-1. [DOI] [PubMed] [Google Scholar]
- 54.Damaj G, Ivanoff S, Coso D, et al. Concomitant systemic and central nervous system non-Hodgkin lymphoma: the role of consolidation in terms of high dose therapy and autologous stem cell transplantation. A 60-case retrospective study from LYSA and the LOC network. Haematologica. 2015;100(9):1199–1206. doi: 10.3324/haematol.2015.126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perry C, Ben Barouch S, Goldschmidt N, et al. Characteristics, management and outcome of DLBCL patients, presenting with simultaneous systemic and CNS disease at diagnosis: a retrospective multicenter study. Am J Hematol. 2019;94(9):992–1001. doi: 10.1002/ajh.25558. [DOI] [PubMed] [Google Scholar]
- 56.Nijland M, Jansen A, Doorduijn JK, Enting RH, Bromberg JEC, Kluin-Nelemans HC. Treatment of initial parenchymal central nervous system involvement in systemic aggressive B-cell lymphoma. Leuk Lymphoma. 2017;58(9):1–6. doi: 10.1080/10428194.2017.1285026. [DOI] [PubMed] [Google Scholar]
- 57.Fleming M, Huang Y, Dotson E, et al. Feasibility of high-dose methotrexate administered on day 1 of (R)CHOP in aggressive non-Hodgkin lymphomas. Blood Adv. 2022;6(2):460–472. doi: 10.1182/bloodadvances.2021005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleming M, Huang Y, Dotson E, et al. Outcomes of patients with diffuse large B-cell and high-grade B-cell lymphomas with synchronous CNS and systemic involvement at diagnosis treated with high-dose methotrexate and R-CHOP: a single-center retrospective study. Ther Adv Hematol. 2022;13 doi: 10.1177/20406207221112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maziarz RT, Wang Z, Zhang MJ, et al. Autologous haematopoietic cell transplantation for non-Hodgkin lymphoma with secondary CNS involvement. Br J Haematol. 2013;162(5):648–656. doi: 10.1111/bjh.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akin S, Hosing C, Khouri I, et al. Autologous stem cell transplantation for large B-cell lymphoma with secondary central nervous system involvement. Blood Adv. 2022;6(7):2267–2274. doi: 10.1182/bloodadvances.2021005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khwaja J, Kirkwood AA, Isbell LK, et al. International multicentre retrospective analysis of thiotepa-based autologous stem cell transplantation for secondary central nervous system lymphoma. Haematologica. 2023;108(3):882–888. doi: 10.3324/haematol.2022.281640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiraga S, Arita N, Ohnishi T, et al. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg. 1999;91(2):221–230. doi: 10.3171/jns.1999.91.2.0221. [DOI] [PubMed] [Google Scholar]
- 63.DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635–643. doi: 10.1200/JCO.1992.10.4.635. [DOI] [PubMed] [Google Scholar]
- 64.Alderuccio JP, Olszewski AJ, Evens AM, et al. HIV-associated Burkitt lymphoma: outcomes from a US-UK collaborative analysis. Blood Adv. 2021;5(14):2852–2862. doi: 10.1182/bloodadvances.2021004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreri AJM, Cattaneo C, Lleshi A, et al. A dose-dense short-term therapy for human immunodeficiency virus/acquired immunodeficiency syndrome patients with high-risk Burkitt lymphoma or high-grade B-cell lymphoma: safety and efficacy results of the "CARMEN" phase II trial. Br J Haematol. 2021;192(1):119–128. doi: 10.1111/bjh.17188. [DOI] [PubMed] [Google Scholar]
- 66.Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–259. doi: 10.1016/S2352-3026(15)00074-5. [DOI] [PubMed] [Google Scholar]
- 67.Ferreri AJ, Guerra E, Regazzi M, et al. Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer. 2004;90(2):353–358. doi: 10.1038/sj.bjc.6601472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Calle N, Isbell LK, Cwynarski K, Schorb E. Advances in treatment of elderly primary central nervous system lymphoma. Br J Haematol. 2022;196(3):473–487. doi: 10.1111/bjh.17799. [DOI] [PubMed] [Google Scholar]
- 69.Schorb E, Isbell L, Kerkhoff A, et al. High-dose chemotherapy and autologous stem cell transplant in elderly and fit primary CNS lymphoma patients - a multicenter study by the Cooperative PCNSL Study Group (MARTA study) Blood. 2022;140(suppl 1):1773–1774. [Google Scholar]
- 70.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113(17):3896–3902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 71.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046–1052. doi: 10.1093/annonc/mdp432. [DOI] [PubMed] [Google Scholar]
- 72.Tai WM, Chung J, Tang PL, et al. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol. 2011;90(7):809–818. doi: 10.1007/s00277-010-1150-7. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto W, Tomita N, Watanabe R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol. 2010;85(1):6–10. doi: 10.1111/j.1600-0609.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 74.Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013;31(25):3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Illerhaus G, Müller F, Feuerhake F, Schäfer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93(1):147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- 77.Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127(13):1642–1649. doi: 10.1182/blood-2015-10-636340. [DOI] [PubMed] [Google Scholar]
- 78.Houillier C, Taillandier L, Dureau S, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37(10):823–833. doi: 10.1200/JCO.18.00306. [DOI] [PubMed] [Google Scholar]
- 79.Houillier C, Dureau S, Taillandier L, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients age 60 years and younger: long-term results of the randomized phase II PRECIS study. J Clin Oncol. 2022;40(32):3692–3698. doi: 10.1200/JCO.22.00491. [DOI] [PubMed] [Google Scholar]
- 80.Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510–e523. doi: 10.1016/S2352-3026(17)30174-6. [DOI] [PubMed] [Google Scholar]
- 81.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Illerhaus G, Ferreri AJM, Binder M, et al. Effects on survival of non-myeloablative chemoimmunotherapy compared to high-dose chemotherapy followed by autologous stem cell transplantation (HDC-ASCT) as consolidation therapy in patients with primary CNS lymphoma - results of an international randomized phase III trial (MATRix/IELSG43) Blood. 2022;140(suppl 2) LBA-3. [Google Scholar]
- 83.Bromberg JE, Doorduijn JK, Illerhaus G, et al. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation--an International Primary Central Nervous System Lymphoma Study Group project. Haematologica. 2013;98(5):808–813. doi: 10.3324/haematol.2012.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 85.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siddiqi T, Wang X, Blanchard MS, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021;5(20):4059–4063. doi: 10.1182/bloodadvances.2020004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alcantara M, Houillier C, Blonski M, et al. CAR T-cell therapy in primary central nervous system lymphoma: the clinical experience of the French LOC network. Blood. 2022;139(5):792–796. doi: 10.1182/blood.2021012932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130(21):2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu K, Wang Y, Teng X, Hu Y, Huang H. Cell subsets and cytokine dynamics in cerebrospinal fluid after CAR-T cell therapy for B-cell acute lymphoblastic leukemia with central nervous system involvement. Bone Marrow Transplant. 2021;56(12):3088–3090. doi: 10.1038/s41409-021-01471-y. [DOI] [PubMed] [Google Scholar]
- 90.Berger SC, Fehse B, Akyüz N, et al. Molecular monitoring of T-cell kinetics and migration in severe neurotoxicity after real-world CD19-specific chimeric antigen receptor T cell therapy. Haematologica. 2023;108(2):444–456. doi: 10.3324/haematol.2022.281110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frigault MJ, Dietrich J, Gallagher K, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022;139(15):2306–2315. doi: 10.1182/blood.2021014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–866. doi: 10.1182/blood.2019001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacobson CA, Falvey C, Bouvier R, et al. A pilot study of axicabtagene ciloleucel (axi-cel) for the treatment of relapsed/refractory primary and secondary central nervous system lymphoma (CNSL) Blood. 2022;140(suppl 1):1060–1061. [Google Scholar]
- 94.Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoang-Xuan K, Houot R, Soussain C, et al. First results of the acsé pembrolizumab phase II in the primary CNS lymphoma (PCNSL) cohort. Blood. 2020;136(suppl 1):15–16. [Google Scholar]
- 97.Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. 2019;37(6):481–489. doi: 10.1200/JCO.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frigault MJ, Armand P, Redd RA, et al. PD-1 blockade for diffuse large B-cell lymphoma after autologous stem cell transplantation. Blood Adv. 2020;4(1):122–126. doi: 10.1182/bloodadvances.2019000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595–1607. doi: 10.1182/bloodadvances.2017014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective 'proof of concept' phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA) Ann Oncol. 2019;30(4):621–628. doi: 10.1093/annonc/mdz032. [DOI] [PubMed] [Google Scholar]
- 101.Grommes C, Wolfe J, Gavrilovic I, et al. Phase II of single-agent ibrutinib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL) Blood. 2018;132(suppl 1):2965. [Google Scholar]
- 102.Zhang Y, Li Y, Zhuang Z, et al. Preliminary evaluation of zanubrutinib-containing regimens in DLBCL and the cerebrospinal fluid distribution of zanubrutinib: a 13-case series. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.760405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deng L, He T, Mei D, et al. Phase II study of zanubrutinib in combination with rituximab and methotrexate, followed by zanubrutinib maintenance in patients with secondary central nervous system lymphoma. Blood. 2022;140(suppl 1):9459–9460. [Google Scholar]
- 104.El-Galaly TC, Cheah CY, Bendtsen MD, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer. 2018;93:57–68. doi: 10.1016/j.ejca.2018.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 106.Bennani NN, Maurer MJ, Nastoupil LJ, et al. Experience with axicabtagene ciloleucel (axi-cel) in patients with secondary CNS involvement: results from the US Lymphoma CAR T Consortium. Blood. 2019;134(suppl 1):763. [Google Scholar]
- 107.Ahmed G, Hamadani M, Shah NN. CAR T-cell therapy for secondary CNS DLBCL. Blood Adv. 2021;5(24):5626–5630. doi: 10.1182/bloodadvances.2021005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghafouri S, Timmerman J, Larson S, Mead MD. Axicabtagene ciloleucel CAR T-cell therapy for relapsed/refractory secondary CNS non-Hodgkin lymphoma: comparable outcomes and toxicities, but shorter remissions may warrant alternative consolidative strategies? Bone Marrow Transplant. 2021;56(4):974–977. doi: 10.1038/s41409-020-01099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karschnia P, Rejeski K, Winkelmann M, et al. Toxicities and response rates of secondary CNS lymphoma after adoptive immunotherapy with CD19-directed chimeric antigen receptor T cells. Neurology. 2022;98(21):884–889. doi: 10.1212/WNL.0000000000200608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 111.Cook MR, Dorris CS, Makambi KH, et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients. Blood Adv. 2023;7(1):32–39. doi: 10.1182/bloodadvances.2022008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sterling CH, Tsai HL, Holdhoff M, et al. Allogeneic blood or marrow transplantation with nonmyeloablative conditioning and high-dose cyclophosphamide-based graft-versus-host disease prophylaxis for secondary central nervous system lymphoma. Transplant Cell Ther. 2021;27(10):863.e1–863.e5. doi: 10.1016/j.jtct.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 114.Samra B, Khoury JD, Morita K, et al. Long-term outcome of hyper-CVAD-R for Burkitt leukemia/lymphoma and high-grade B-cell lymphoma: focus on CNS relapse. Blood Adv. 2021;5(20):3913–3918. doi: 10.1182/bloodadvances.2021004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hill QA, Owen RG. CNS prophylaxis in lymphoma: who to target and what therapy to use. Blood Rev. 2006;20(6):319–332. doi: 10.1016/j.blre.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Nkrumah FK, Perkins IV. Relapse in Burkitt's lymphoma. Int J Cancer. 1976;17(4):455–460. doi: 10.1002/ijc.2910170407. [DOI] [PubMed] [Google Scholar]
- 117.Olszewski AJ, Jakobsen LH, Collins GP, et al. Burkitt lymphoma International Prognostic Index. J Clin Oncol. 2021;39(10):1129–1138. doi: 10.1200/JCO.20.03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13(8):1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 119.Noy A, Lee JY, Cesarman E, et al. AMC 048: modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood. 2015;126(2):160–166. doi: 10.1182/blood-2015-01-623900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med. 2013;369(20):1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 122.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial) Blood. 2008;112(6):2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chamuleau M, Stenner F, Chitu D, et al. R-CODOX-M/R-IVAC versus DA-EPOCH-R in patients with newly diagnosed high-risk Burkitt lymphoma; first results of a multi-center randomized HOVON/SAKK trial. Paper presented at: European Hematology Association Congress; 9-12 June 2022, Vienna, Austria. https://library.ehaweb.org/eha/2022/eha2022-congress/366213/med.chamuleau.r-codox-m.r-ivac.versus.dose-adjusted28da29-epoch-r.in.patients.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Ace_id%3D2233%2Amarker%3D1750%2Afeatured%3D17676
- 124.Evens AM, Danilov A, Jagadeesh D, et al. Burkitt lymphoma in the modern era: real-world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137(3):374–386. doi: 10.1182/blood.2020006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manji F, Chow E, Gerrie AS, et al. Outcomes in relapsed/refractory Burkitt lymphoma: a multi-centre Canadian experience. Blood. 2021;138(suppl 1):2525. [Google Scholar]
- 126.Liu Y, Deng B, Hu B, et al. Sequential different B-cell antigen-targeted CAR T-cell therapy for pediatric refractory/relapsed Burkitt lymphoma. Blood Adv. 2022;6(3):717–730. doi: 10.1182/bloodadvances.2021004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McLaughlin N, Wang Y, Witzig T, et al. Central nervous system involvement by mantle cell lymphoma. Leuk Lymphoma. 2023;64(2):371–377. doi: 10.1080/10428194.2022.2148211. [DOI] [PubMed] [Google Scholar]
- 128.Tucker DL, Naylor G, Kruger A, Hamilton MS, Follows G, Rule SA. Ibrutinib is a safe and effective therapy for systemic mantle cell lymphoma with central nervous system involvement - a multi-centre case series from the United Kingdom. Br J Haematol. 2017;178(2):327–329. doi: 10.1111/bjh.14122. [DOI] [PubMed] [Google Scholar]
- 129.Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695–1698. doi: 10.1182/blood-2015-05-647834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robbins DW, Noviski M, Rountree R, et al. Nx-5948, a selective degrader of BTK with activity in preclinical models of hematologic and brain malignancies. Blood. 2021;138(suppl 1):2251. [Google Scholar]
- 131.Chirnomas D, Hornberger KR, Crews CM. Protein degraders enter the clinic - a new approach to cancer therapy. Nat Rev Clin Oncol. 2023;20(4):265–278. doi: 10.1038/s41571-023-00736-3. [DOI] [PubMed] [Google Scholar]
- 132.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023;41(14):2594–2606. doi: 10.1200/JCO.22.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41(12):2238–2247. doi: 10.1200/JCO.22.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dickinson MJ, Carlo-Stella C, Morschhauser F, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387(24):2220–2231. doi: 10.1056/NEJMoa2206913. [DOI] [PubMed] [Google Scholar]
- 136.Bartlett NL, Assouline S, Giri P, et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2023;7(17):4926–4935. doi: 10.1182/bloodadvances.2022009260. [DOI] [PMC free article] [PubMed] [Google Scholar]