Abstract
Introduction
Familial glucocorticoid deficiency (FGD) is a rare autosomal recessive disease resulting from isolated glucocorticoid deficiency or unresponsiveness to adrenocorticotropic hormone. Patients with FGD usually present in infancy or early childhood with hyperpigmentation, recurrent infections, and hypoglycemia. The salt-wasting crisis is rare.
Case Presentation
A term female neonate was admitted to the neonatal intensive care unit due to respiratory distress. On physical examination, she had generalized hyperpigmentation. Initial laboratory work-up yielded normal serum electrolytes and glucose. Hyponatremia and hyperkalemia emerged on follow-up. The patient was diagnosed as having primary adrenal insufficiency (PAI) with elevated plasma adrenocorticotropin hormone and reduced cortisol levels and hydrocortisone. We started on oral sodium (5 mEq/kg/day) and fludrocortisone (FC) (0.2 mg/day) treatment to the patient. Ultrasonography revealed hypoplastic adrenal glands. Molecular genetic analysis revealed a previously reported homozygous pathogenic variant NM_000529.2: c.560delT (p.V187fs*29) in the MC2R gene. FC dose was tapered to 0.05 mg/day on the third month of life and was stopped at tenth months of age with maintenance of normal serum electrolytes and clinical findings.
Conclusion
FGD due to MC2R gene mutation may rarely present with a salt-wasting crisis in the neonatal period. Identifying the causative gene with the pathogenic variant in PAI may serve to individualize a treatment plan.
Keywords: Autosomal recessive, Familial glucocorticoid deficiency, Hyponatremia, MC2R gene, Newborn
Established Facts
-
•
Familial glucocorticoid deficiency is an autosomal recessive disorder, also known as isolated glucocorticoid deficiency or unresponsiveness to ACTH. Patients usually present with hypoglycemia, hyperpigmentation, and recurrent infections. The hyponatremia is rare.
Novel Insights
-
•
This is the report of a patient with a homozygous pathogenic variant c.560delT (p.V187fs*29) of the MC2R gene who presented with salt-wasting crisis in the neonatal period and was successfully halted from mineralocorticoid treatment before 1 year of age, thereby preventing the risk of hypertension.
Introduction
Adrenocorticotropin hormone (ACTH) is a polypeptide hormone that is produced and secreted from the anterior pituitary gland in response to biological stress. ACTH binds to the melanocortin-2 receptor (MC2R) which is mainly expressed in adrenocortical cells of the adrenal cortex and stimulates cortisol production [Yang et al., 2015].
Familial glucocorticoid deficiency (FGD) (OMIM #202200) is an autosomal recessive disorder, also known as isolated glucocorticoid deficiency or inherited unresponsiveness to ACTH. While there are multiple subtypes of FGD, the two most common forms are type 1 (OMIM 607397) and type 2 (OMIM 609196). While mutations in MC2R cause type 1, melanocortin-2 receptor helper protein (MRAP) mutations cause type 2 FGD [Mohammed et al., 2022]. FGD was first reported in 1959 in two sisters by Shepard et al. [Shepard et al., 1959]. Low plasma cortisol, high plasma ACTH, and normal mineralocorticoid levels are frequently reported on laboratory work-up of patients with pathogenic variants in the MC2R gene. These patients usually present with hyperpigmentation, recurrent infections, hypoglycemic seizures, and coma [Mohammed et al., 2022]. Salt-wasting crises are rarely reported in patients with MC2R pathogenic variants. Herein, we present a newborn who was hospitalized in our neonatal intensive care unit due to respiratory distress, developed electrolyte imbalance in follow-up, was diagnosed with primary adrenal insufficiency (PAI), and a homozygous pathogenic variant in the MC2R gene was identified on subsequent genetic work-up [Guran et al., 2016].
Case Report
A female baby was born at 42 weeks of gestation via normal vaginal delivery with a birth weight of 3,740 g (−0.2 standard deviation) from a 37-year-old mother who had an uneventful regular antenatal follow-up. The baby was intubated in the delivery room and admitted to the neonatal intensive care unit. On physical examination, the baby had generalized hyperpigmentation and no dysmorphic appearance or organomegaly. Anthropometric measurements revealed length: 52 cm (−0.24 standard deviation), head circumference: 38.2 cm (1.75 standard deviation). The genital examination was normal. The parents were first-degree cousins. She had two healthy siblings.
Echocardiographic assessment owing to prolonged respiratory distress revealed pulmonary hypertension. Laboratory work-up on day 1 of life (DOL) revealed complete blood count in normal ranges, serum sodium level: 141 mmol/L (range: 135–145 mmol/L), and potassium: 4.1 mmol/L (range: 3.5–4.5 mmol/L). Sedation, hydration, antibiotics, inhaled nitric oxide, and inotropes were commenced. Invasive ventilation support, inhaled nitric oxide therapy, and inotropic support were gradually tapered and discontinued. On DOL 4 laboratory work-up revealed venous blood glucose: 56 mg/dL, sodium: 122 mmol/L, potassium: 5.6 mmol/L, venous pH: 7.34 PCO2: 47 mm Hg, and HCO3:24 mmol/L. Persistent hyponatremia on follow-up prompted pediatric endocrinology consultation. On DOL 7, further laboratory work-up revealed high plasma ACTH: 1,643 ng/L (range: 7.2–63.3 ng/L), low plasma cortisol: 0.09 µg/dL (range: 6–18.4 µg/dL), low plasma 17-hydroxy-progesterone: <0.04 µg/L (range: 0.07–1.06 µg/L), normal plasma androstenedione: 0.16 µg/L (range: 0.14–3.45 µg/L), normal plasma aldosterone: 29.5 ng/dL (range: 6–179 ng/dL), and plasma renin activity was 5.8 ng/ml/h (range: 2.4–37 ng/mL/h). Moreover, thyroid function tests revealed increased serum TSH: 14.9 mU/L (range: 0.72–11 mU/L) and reduced serum-free T4: 6.2 ng/L (range: 8.9–22 ng/L).
The baby was diagnosed with PAI and congenital primary hypothyroidism (PH). Ultrasonography revealed bilateral hypoplastic adrenal glands (6 × 3 mm) and eutopic thyroid with normal volume. On DOL 10, the patient was started on intravenous hydrocortisone (HC) at a stress dose of 50 mg/m2/day, fludrocortisone (FC) 0.2 mg/day, oral sodium 5 mEq/kg/day, and levothyroxine 10 μg/kg/day. The patient’s vital signs and electrolyte values were normal in the follow-up, and the HC dose was reduced to 15 mg/m2/day.
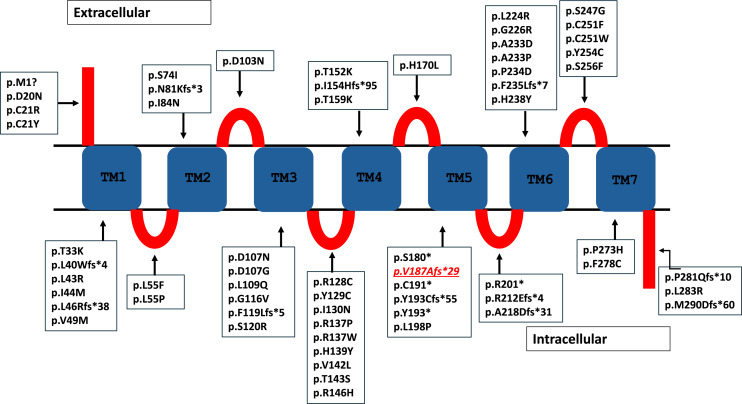
After receiving informed consent from the parents, molecular genetic analysis for adrenal insufficiency revealed a homozygous pathogenic variant NM_000529.2: c.560delT (p.V187fs*29) in the MC2R gene. This variant is pathogenic (PVS1, PM2, PP5) according to ACMG criteria. All variations in the MC2R gene reported in the literature so far are shown in Figure 1.
Fig. 1.
Cartoon figure of the MC2R protein. The variations found so far in the MC2R gene are shown in here. The variant found in this patient is indicated by the underlined and italicized text. TM, transmembrane domain; red blocks, extracellular and intracellular domains.
Protein Homology Modeling for c.560delT (p.V187fs*29) Variant in the MC2R Gene
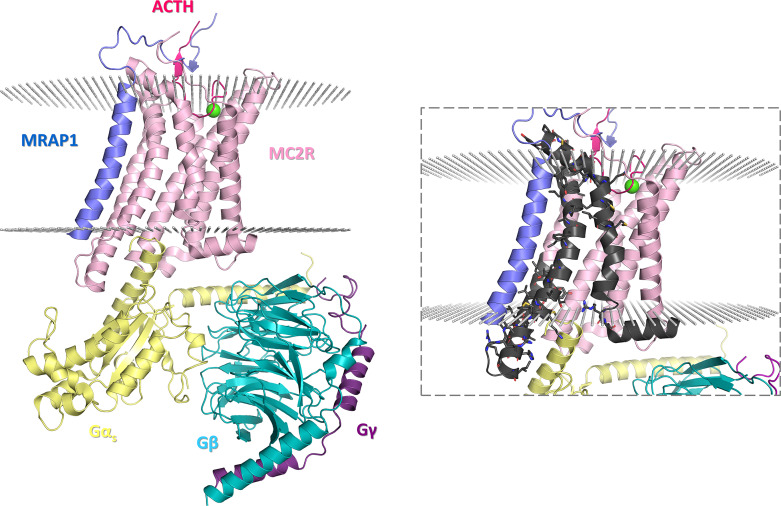
The cryo-electron microscopy structure of the ACTH-bound MC2R in complex with melanocortin receptor accessory protein 1 (MRAP1) and the Gs protein (resolution: 3.30 Å; aggregation state: particle; reconstruction method: single particle) clearly indicates that MC2R folds into a seven-transmembrane (7TM) domain [Luo et al., 2023]. The same structure also reveals that ACTH is embedded within an external pocket formed by the TM1 (residues 28–47), TM2 (residues 61–86), TM3 (residues 100–126), and TM4 (residues 147–172) segments of MC2R as well as the extracellular loop 3, ECL3, between TM6 (residues 215–241) and TM7 (residues 257–279) and that the regulatory protein MRAP1 lies parallel to and is engaged in multiple polar contacts with the TM5 (residues 173–195) and TM6 segments of MC2R. Interestingly, ACTH appears to be further secured within the external pocket of MC2R by the N-terminus of MRAP1 acting as a “seatbelt.” The p.V187fs*29 alteration causes mistranslation onward from Val187, affecting the second half of TM5, TM6, and TM7 (Fig. 2). Therefore, in the context of the predicted molecular phenotype of the individual case based on the MC2R structure-function relationship, p.V187fs*29 can be interpreted as a pathogenic mutation due to the fact that it not only disrupts the canonical 7TM fold of the receptor, but that it impairs interactions of the receptor with both MRAP1 and ACTH. While ACTH is the natural agonist of MC2R-stimulating glucocorticoid biogenesis and cortisol release during stress [Russell and Lightman, 2019], MRAP1 is responsible for the formation of a biologically active receptor [Rouault et al., 2017] and stabilization of ACTH-MC2R binding [Luo et al., 2023]. In the light of our computational findings, the p.V187fs*29 alteration caused by thus identified c.560delT variant corroborates with the molecular etiology of FGD.
Fig. 2.
The molecular architecture of the ACTH-MC2R-Gs-MRAP1 complex (PDB ID: 8GY7). Individual subunits are represented by cartoons in varying colors (ACTH, bright pink; MC2R, lilac; Gαs, yellow; Gβ, turquoise; Gγ, purple; MRAP1, blue), the lipid bilayer is represented by small spheres in light gray, and a single calcium ion is represented by a large sphere in green. The inset shows the relevant portion of MC2R affected by the p.V187fs*29 alteration (black). Sticks correspond to the interfacial residues on the surface of the canonically translated C-terminal fragment of MC2R, which interact with other structural elements in the complex. The figures were rendered by using the PyMOL Molecular Graphics System, version 1.8 (Schrödinger LLC, Portland, OR, USA).
The patient was discharged on DOL 38 with HC 15 mg/m2/day, FC 0.2 mg/day, levothyroxine 25 μg/day, and oral sodium 5 mEq/kg/day. On follow-up, the FC dose was tapered to 0.05 mg/day, and continued follow-up revealed normal serum electrolytes and appropriate growth. Her last anthropometric measurements in the seventh month of life were normal. Genetic counseling was provided to the parents.
Genetic Testing
Genomic DNA was isolated from peripheral blood leukocytes using a QIAamp DNA Blood Mini QIAcube Kit (Qiagen, Hilden, Germany), according to the manufacturers’ protocols. Genomic DNA was fragmented to 400 bp using QIAseq® FX DNA Library Kits (Qiagen). After fragmentation, the libraries were prepared using the QIAseq® Human Exome Kit as per the manufacturer’s instructions (Qiagen). Paired-end sequencing (150 bp) was performed on the NovaSeq 6000 system according to the manufacturer’s guidelines (Illumina Inc., San Diego, CA, USA). Raw data were analyzed through the “QIAGEN Clinical Insight (QCI) Interpret” data analysis platform. To determine pathogenic variants, we filtered in: (1) all nonsynonymous, missense, nonsense, frameshift, splice site, nostop, no-start, indels, and inframe variants in all protein-coding genes, (2) synonymous or intronic variants affecting the consensus splice sites, (3) variants with minor allele frequency <1.0% in population studies (1,000 Genome [1,000 G], Genome Aggregation Database [gnomAD]), (4) variants with variant frequency is between 20 and 100%. To evaluate the pathogenicity of variants, in silico prediction tools (DANN, DEOGEN2, EIGEN, MVP, MutationAssessor, PrimateAI, REVEL, SIFT, PROVEAN, LRT, GERP, FATHMM-MKL, and MutationTaster), segregation analysis, allele frequencies in population studies (1000 G, gnomAD, ExAC), and American College of Medical Genetics and Genomics (ACMG) criteria were used [Richards et al., 2015]. The genomic build numbers of the primers used for Sanger sequencing are as follows: forward primer genomic build number: Chr 18:13885313-13885335 and reverse primer genomic build number: Chr 18: 13884796-13884773 (GRCh38).
Discussion
We reported a neonate presenting with salt-wasting crises and hypoplastic adrenal glands due to a pathogenic homozygous variant in the MC2R gene [Guran et al., 2016]. This is a very rare presentation of PAI due to pathogenic variants in the MC2R gene since it usually presents with hypoglycemia in the late neonatal period. While congenital adrenal hyperplasia (CAH) due to 21 hydroxylase deficiency is the most common genetic cause of PAI, the clinical diagnosis in non-CAH patients in the neonate might be challenging owing to a more insidious course and not being covered in the neonatal-screening program.
Genetic causes of adrenal insufficiency in children can be classified into four main groups; impaired steroidogenesis, adrenal hypoplasia, FGD/FGD-like disorders, and adrenal destruction [Guran et al., 2016; Uçar et al., 2016]. Laboratory work-up of our patient with hypoplastic adrenal glands revealed FGD1 as the ultimate diagnosis. Pathogenic variants in genes associated with adrenal hypoplasia and PAI include CYP11A1, DAX1, MC2R, and SF1 as described before [Guran et al., 2016]. Children with FGD1 typically present with symptoms and signs of glucocorticoid deficiency such as hypoglycemic convulsions, tall stature, hyperpigmentation, prolonged jaundice, and sepsis [Clark and Weber, 1998; Clark et al., 2005]. Tall stature in FGD has been linked to extremely high levels of ACTH, which acts via other melanocortin receptors to affect bone and cartilage growth independent of growth hormone [Elias et al., 2000]. Five members of the melanocortin receptor family (MC1R to MC5R) are expressed to varying degrees in bone, and high ACTH levels acting on these receptors end up in tall stature [Zhong et al., 2005]. However, the height of our patient was normal.
The association of disorders in the renin-angiotensin system, aldosterone synthesis, or sodium homeostasis with FGD1 has not been proven [Lin et al., 2007]. Patients with classical FGD1 do not lose salt and do not need mineralocorticoids. MC2R is also expressed in the zona glomerulosa of the adrenal gland, and administration of ACTH analog to humans results in the release of aldosterone [Xia and Wikberg, 1996; Arvat et al., 2000]. Transient hyponatremia was reported in a couple of children with severe disruption of the receptor [Lin et al., 2007; Chan et al., 2009]. Our patient required mineralocorticoid replacement as well, albeit at lower doses than those suggested in the neonatal and infantile period considering the physiological mineralocorticoid resistance in this period of life. We tapered the dose of FC to 0.05 mg/day in the third month of life with no remarkable alteration in serum electrolyte levels. A trial of cessation of mineralocorticoid treatment in such cases owing to restoration of mineralocorticoid response in the second half of infancy and thereafter is recommended [Lin et al., 2007; Gao and Chen, 2020]. We stopped FC treatment at tenth months of age with continued maintenance of normal electrolyte levels.
She was also diagnosed with congenital eutopic PH with high serum TSH and low serum-free T4 levels and was placed on l-thyroxine replacement. Due to the presence of a eutopic thyroid gland with normal volume, longitudinal follow-up will determine whether it is transient or permanent. There was no genotype-phenotype association in a couple of cases with congenital PH and pathogenic homozygous variants in the MC2R gene in the literature [Al Kandari et al., 2011; Mathew and Kovacs, 2011; Heshmatzad et al., 2020]. Evidence from animal studies suggests that glucocorticoid deficiency induces TSH secretion and steroid replacement therapy normalizes serum TSH levels [Barnett et al., 1982]. However, subclinical hypothyroidism was shown to persist in some cases with MC2R following cessation of l-thyroxin treatment [Al Kandari et al., 2011]. The existence of a potential association of pathogenic variants in the MC2R gene with thyroid dysfunction remains conflicting. Our patient is also receiving l-thyroxine treatment.
To conclude, our case highlights the need to identify the genetic cause of PAI with adrenal hypoplasia to individualize the follow-up and treatment as needed. The coexistence of congenital PH in some patients with pathogenic variants in the MC2R gene remains to be further investigated.
Statement of Ethics
Written informed consent was obtained from the patient’s parents for publication of the details of her medical case, accompanying data, and investigations. Ethical approval was not required for this study in accordance with local and national regulations.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding for this study.
Author Contributions
Aysenur Kardas Yildiz and Ahmet Ucar wrote the manuscript. Ayberk Turkyilmaz and Kerem Terali worked on genetic part of study. Ali Bulbul, Buse Ozer Bekmez, and Aydilek Dagdeviren Cakir provided clinical evaluation. All authors reviewed and approved the final manuscript.
Funding Statement
There was no funding for this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, A.K.Y., upon reasonable request.
References
- Arvat E, Di Vito L, Lanfranco F, Maccario M, Baffoni C, Rossetto R, et al. Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. J Clin Endocrinol Metab. 2000;85(9):3141–6. [DOI] [PubMed] [Google Scholar]
- Al Kandari HM, Katsumata N, al Alwan I, al Balwi M, Rasoul MS. Familial glucocorticoid deficiency in five Arab kindreds with homozygous point mutations of the ACTH receptor (MC2R): genotype and phenotype correlations. Horm Res Paediatr. 2011;76(3):165–71. [DOI] [PubMed] [Google Scholar]
- Barnett AH, Donald RA, Espiner EA. High concentrations of thyroid-stimulating hormone in untreated glucocorticoid deficiency: indication of primary hypothyroidism? Br Med J. 1982;285(6336):172–3. 10.1136/bmj.285.6336.172-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Weber A. Adrenocorticotropin insensitivity syndromes. Endocr Rev. 1998;19(6):828–43. 10.1210/edrv.19.6.0351. [DOI] [PubMed] [Google Scholar]
- Clark AJ, Metherell LA, Cheetham ME, Huebner A. Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol Metab. 2005;16(10):451–7. 10.1016/j.tem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chan LF, Metherell LA, Krude H, Ball C, O’Riordan SM, Costigan C, et al. Homozygous nonsense and frameshift mutations of the ACTH receptor in children with familial glucocorticoid deficiency (FGD) are not associated with long-term mineralocorticoid deficiency. Clin Endocrinol. 2009;71(2):171–5. 10.1111/j.1365-2265.2008.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LL, Huebner A, Metherell LA, Canas A, Warne GL, Bitti ML, et al. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol. 2000;53(4):423–30. 10.1046/j.1365-2265.2000.01122.x. [DOI] [PubMed] [Google Scholar]
- Guran T, Buonocore F, Saka N, Ozbek MN, Aycan Z, Bereket A, et al. Rare causes of primary adrenal insufficiency: genetic and clinical characterization of a large nationwide cohort. J Clin Endocrinol Metab. 2016;101(1):284–92. 10.1210/jc.2015-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chen L. Primary adrenocortical insufficiency case series in the neonatal period: genetic etiologies are more common than expected. Front Pediatr. 2020;8:464. 10.3389/fped.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmatzad K, Mahdieh N, Rabbani A, Didban A, Rabbani B. The genetic perspective of familial glucocorticoid deficiency: in SilicoAnalysis of two novel variants. Int J Endocrinol. 2020;2020:2190508. 10.1155/2020/2190508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Hindmarsh PC, Metherell LA, Alzyoud M, Al-Ali M, Brain CE, et al. Severe loss-of-function mutations in the adrenocorticotropin receptor (ACTHR, MC2R) can be found in patients diagnosed with salt-losing adrenal hypoplasia. Clin Endocrinol. 2007;66(2):205–10. 10.1111/j.1365-2265.2006.02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Feng W, Ma S, Dai A, Wu K, Chen X, et al. Structural basis of signaling regulation of the human melanocortin-2 receptor by MRAP1. Cell Res. 2023;33(1):46–54. 10.1038/s41422-022-00751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RP, Kovacs WJ. Short stature in a patient with familial glucocorticoid deficiency. J Pediatr Endocrinol Metab. 2011;24(7-8):569–71. 10.1515/jpem.2011.203. [DOI] [PubMed] [Google Scholar]
- Mohammed I, Haris B, Hussain K. A novel homozygous MC2R variant leading to type-1 familial glucocorticoid deficiency. J Endocr Soc. 2022;6(6):bvac058. 10.1210/jendso/bvac058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–24. 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault AAJ, Srinivasan DK, Yin TC, Lee AA, Sebag JA. Melanocortin receptor accessory proteins (MRAPs): functions in the melanocortin system and beyond. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt A):2462–7. 10.1016/j.bbadis.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525–34. 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- Shepard TH, Landing BH, Mason DG. Familial Addison’s disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA J Dis Child. 1959;97(2):154–62. 10.1001/archpedi.1959.02070010156002. [DOI] [PubMed] [Google Scholar]
- Uçar A, Baş F, Saka N. Diagnosis and management of pediatric adrenal insufficiency. World J Pediatr. 2016;12(3):261–74. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wikberg JE. Localization of ACTH receptor mRNA by in situ hybridization in mouse adrenal gland. Cell Tissue Res. 1996;286(1):63–8. 10.1007/s004410050675. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mishra V, Crasto CJ, Chen M, Dimmitt R, Harmon CM. Third transmembrane domain of the adrenocorticotropic receptor is critical for ligand selectivity and potency. J Biol Chem. 2015;290(12):7685–92. 10.1074/jbc.M114.596122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, et al. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36(5):820–31. 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, A.K.Y., upon reasonable request.