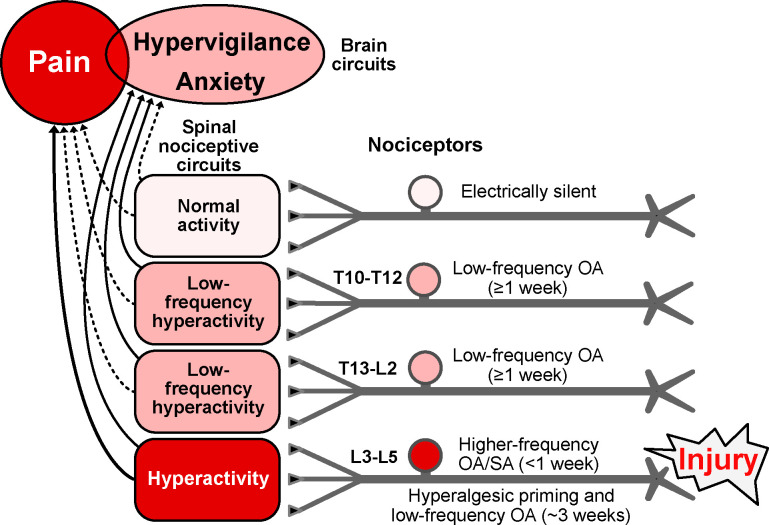
Figure 7.
Hypothesis about functions of nociceptor OA based on effects of hindpaw incision injury found to be retained in vitro and on related findings in vivo (see text). Incision induces extrinsic OA stimulated by humoral factors plus intrinsic SA for several days in nociceptors innervating the injured hindpaw (L3-L5 DRGs), with the nociceptor OA activating pathways in the spinal cord that drive pain and/or hypervigilance/anxiety. When ongoing pain ends, lower-frequency OA continues in L3-L5 nociceptors along with hyperalgesic priming for several weeks. Low-frequency OA occurs during the entire ~3-week period in widespread nociceptors, including those with somata in T13-L2 and T10-T12 DRGs and, if the injury is unilateral, in some contralateral DRGs (not shown). Low-frequency nociceptor OA is proposed to drive general hypervigilance/anxiety more readily than behaviorally significant pain, and to contribute to hyperalgesic priming, especially in the injured region. Higher frequency OA drives ongoing pain as well as anxiety. Red indicates components that drive pain. Pink indicates components with activity subthreshold for driving pain, but which are sufficient to drive hypervigilance/anxiety after hindpaw injury. Solid lines indicate suprathreshold pathways for driving brain-mediated affective states (pain, anxiety, perhaps other negative states); dashed lines are subthreshold. Some electrically silent nociceptors are found in all DRGs after incision injury, but silent nociceptors may be most common in DRGs farthest from the injury. L, lumbar, OA, ongoing activity, T, thoracic.