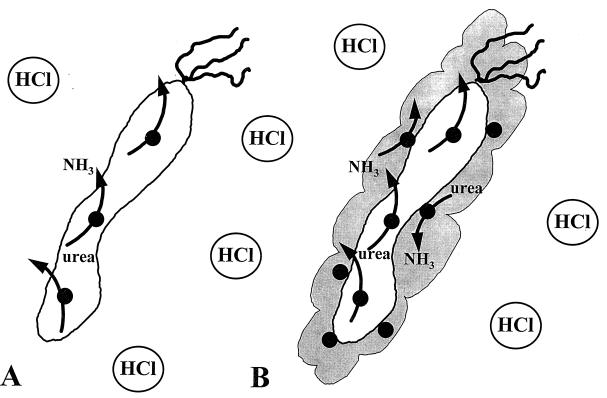
FIG. 7.
Model describing the roles of H. pylori urease activity in the cytoplasmic and surface-associated compartments. H. pylori urease activity exhibits a pH optimum of 8.3. Free urease is rapidly inactivated by exposure to pH <5 (2, 39) and likely does not contribute to acid resistance. (A) Most of the early-logarithmic-phase and some late-logarithmic-phase H. pylori cells contain cytoplasmic urease exclusively, with no surface-associated urease (38). H. pylori cells exhibiting cytoplasmic urease activity only are also generated by inhibition of surface-associated urease with 1 μM flurofamide (38). The cytoplasmic urease degrades urea to produce ammonia, which may be exported but is not sufficient to permit survival at pH 2 for 30 min. (B) In the late logarithmic phase of growth, H. pylori possesses both cytoplasmic and surface-associated urease activity (38), allowing quantitatively more urease activity per bacterium. The surface-associated urease activity is sensitive to flurofamide (38), a poorly diffusible urease inhibitor, and is protected from external low pH due to its association with the molecular chaperonin HspB (heat shock protein B, a groEL homolog) at the cell surface. Rapid external hydrolysis of urea helps to prevent entry of H+ into the bacterial cytoplasm, therefore maintaining neutral-to-alkaline cytoplasmic pH and allowing full urease activity in the cytoplasmic compartment. Internal urease in the absence of external urease is unable to maintain a neutral-to-alkaline cytoplasmic pH for optimum urease activity. It is important to note that the observations presented above are valid for an external pH of 2.0. It remains to be determined whether cytoplasmic urease alone is sufficient to allow survival of H. pylori at a higher external pH (3 to 5).