Abstract
Purpose:
Diagnosis and management of non-infectious uveitis (NIU), a major cause of blindness worldwide, are challenging. Corticosteroids, the cornerstone of therapy, are not appropriate for long-term use, and while non-biologic and biologic immunomodulators may be used for some patients, data on their efficacy and safety in this population are limited. Repository corticotropin injection (RCI), believed to affect uveitis by multiple mechanisms, has received regulatory approval for treatment of ophthalmic diseases including posterior uveitis, but is not widely used or discussed in guidelines for the management of uveitis and ocular inflammatory diseases.
Methods:
The index study employed a modified Delphi process with a panel of 14 US-based ophthalmologists. Consensus recommendations were developed through a series of three questionnaires. Panellists rated statements on a Likert scale from −5 (strongly disagree) to +5 (strongly agree).
Results:
The Delphi panel provided consensus recommendations on examinations and testing needed for diagnosis, treatment goals, and the use of corticosteroids, as well as the use of non-biologic and biologic immunomodulators. The panel reached consensus that RCI may be considered for posterior and pan-uveitis, and dosing should be individualized for each patient. Dose reduction/discontinuation should be considered for excessive RCI-related toxicity, hyperglycaemia and/or diabetic complications, excessive costs, or remission ≥ 2 years. Patients should be weaned from RCI if uveitis is stable and well controlled. Adverse events during RCI therapy can be managed by appropriate interventions, with dose reduction/discontinuation considered if events are severe or recurrent.
Conclusions:
Expert consensus suggests RCI may be an appropriate treatment option for some patients with uveitis when other therapies are ineffective or intolerable.
Keywords: repository corticotropin injection therapy, RCI therapy, non-infectious uveitis, Delphi
Introduction
Uveitis is a leading cause of blindness accounting for as much as 15% of cases worldwide and approximately 10% of permanent vision loss in the United States (Suttorp-Schulten & Rothova 1996; Foster et al. 2016). The incidence is estimated to be approximately 52.4 cases per 100 000 person-years, and prevalence estimates range from 115.3 to 540 cases per 100 000 adults (Gritz & Wong 2004; Thorne et al. 2016; Gonzalez et al. 2018). In the developed world, most uveitis has a non-infectious aetiology thought to result from an aberrant inflammatory immune response that attacks ocular tissue (Caspi 2010). Despite the importance of non-infectious uveitis (NIU) as a cause of blindness, its incidence and prevalence are low enough that it is considered a rare disease (Barisani-Asenbauer et al. 2012). Most cases of NIU are confined to the eye. However, approximately one-third of cases are associated with extraocular autoimmune diseases such as ankylosing spondylitis, Adamantiades–Behçet’s disease, and sarcoidosis (Barisani-Asenbauer et al. 2012).
Early, accurate diagnosis of uveitis is important due to the potential for severe, irreversible visual loss from some forms of the disease, and the need for different treatment approaches depending on the aetiology of the uveitis. Diagnosis of NIU and other forms of uveitis is often challenging, with one study reporting that only 17% of patients received a definitive diagnosis on the first evaluation (Rodriguez et al. 1996). Several strategies to address this difficulty have been published, including differential diagnosis based on the location and type of inflammation and the associated systemic symptoms, (Opremcek 1995) and a process known as ‘name meshing’ based on comparing the patient’s clinical profile with the characteristics of known ocular inflammatory disorders (Smith & Nozik 2003).
Medical management of NIU centres on ocular and oral corticosteroids and a wide range of biologic and non-biologic immunomodulators. Corticosteroids (topical, ocular and/or systemic) are the cornerstone of therapy but are almost always not appropriate for long-term therapy due to their widely known adverse effect profile. If short-term corticosteroid therapy is not sufficient, or in certain conditions warranting early use of immunomodulators, non-biologic or biologic immunomodulatory agents are added and the steroid dose is tapered then discontinued (Foster et al. 2016). However, a survey of US ophthalmologists and rheumatologists who manage patients with uveitis revealed that the majority were not familiar with, or did not adhere to, currently recommended guidelines (Jabs et al. 2000; Gupta & Murray 2006). High corticosteroid doses are all too frequently used to maintain disease control, and awareness of recommended guidelines for the treatment of NIU is low (Nguyen et al. 2011).
A variety of immunomodulatory agents have been used in treating uveitis, although data on their efficacy and safety in uveitis are limited and the only agents with regulatory approval for this indication are adalimumab, approved for uveitis in 2016, and repository corticotropin injection (RCI), introduced in 1952 (AbbVie 2018; Mallinckrodt 2018). Ophthalmologists may not be comfortable managing these medications and their associated adverse effects (e.g. fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioural and mood changes, increased appetite and weight gain for RCI, and infections (including serious infections leading to hospitalization or death), malignancies, injection site reactions, headache and rash for adalimumab), and often refer these aspects of care to rheumatologists, the patient’s primary care practitioner, or other appropriate physicians.
Repository corticotropin injection (RCI), a naturally sourced complex mixture of porcine-derived adrenocorticotropic hormone analogs and other pituitary peptides, stimulates secretion of cortisol and related substances and binds to and activates melanocortin receptors (Catania et al. 2010; Mallinckrodt 2018). Activation of melanocortin receptors may provide both anti-inflammatory and immunomodulatory effects, decrease the activity of helper T lymphocytes, and increase the population and activity of regulatory T lymphocytes (Catania et al. 2010; Taylor & Lee 2011). Although RCI is marketed for the treatment of severe acute and chronic inflammatory processes involving the eye and its adnexa, including diffuse posterior uveitis, it is not widely used and is not discussed in current guidelines (Foster et al. 2016). The authors are aware of only one peer-reviewed publication related to the use of RCI in uveitis, a case study by Agarwal and colleagues in 2016 (Agarwal et al. 2016). Clinical trials of RCI in retinal vasculitis [single-arm open-label study in 40 adults with non-infectious active retinal vasculitis; RCI dose not stated] (NCT03066869 2017), ocular sarcoidosis [single-arm open-label study in 20 adults with sarcoidosis and active uveitis requiring therapy; RCI is to be administered at a dose of 80 units daily for 10 days, and 80 units twice weekly thereafter] (NCT02725177 2016), and proliferative vitreoretinopathy [single-arm open-label study in 15 adults undergoing surgery for retinal detachment due to PVR; RCI is to be administered at a dose of 80 units twice weekly for 8 weeks] (NCT03727776 2018) are underway as of September 2020.
Given the limited clinical evidence available on the use of RCI in uveitis and the lack of relevant guideline recommendations, expert opinion on RCI was considered a valuable resource for clinicians treating uveitis. The authors convened a panel of ophthalmologists experienced in the treatment of uveitis to develop expert consensus recommendations on the diagnosis and treatment of uveitis, and particularly the use of RCI in managing uveitis, using a modified Delphi process. The Delphi process, which dates back to the 1950s, was originally designed to facilitate the development of consensus in the social sciences, particularly for topics where substantial experience and practical knowledge exist but definitive evidence is not available (Delbecq et al. 1975; Schutt et al. 2010; Haines et al. 2013; Mansell et al. 2014; Phillips et al. 2014; Pietersma et al. 2014; Pleyer et al. 2014). Delphi methods have been used to develop several consensus recommendations for specific clinical issues (Hasson et al. 2000; de Meyrick 2003; Hsu & Sandford 2007; Schutt et al. 2010; Rahaghi et al. 2017).
Materials and methods
The study was conceived and moderated by the lead authors (CSF and QDN). Candidates for participation in the Delphi panel were ophthalmologists familiar with the use of RCI in the treatment of uveitis, identified by the moderators and/or using a commercially available prescribing database. Potential panellists confirmed they had used RCI to treat uveitis in at least 5 patients. All panellists who actively participated in the Delphi process and manuscript development are co-authors. Active participation was defined as completing the final Delphi questionnaire and at least one other questionnaire and reviewing, revising, and approving the manuscript at each stage of development.
The survey utilized a modified Delphi process with a sequence of three questionnaires (Fig. 1), circulated via an online survey platform (Surveygizmo.com).
Fig. 1.
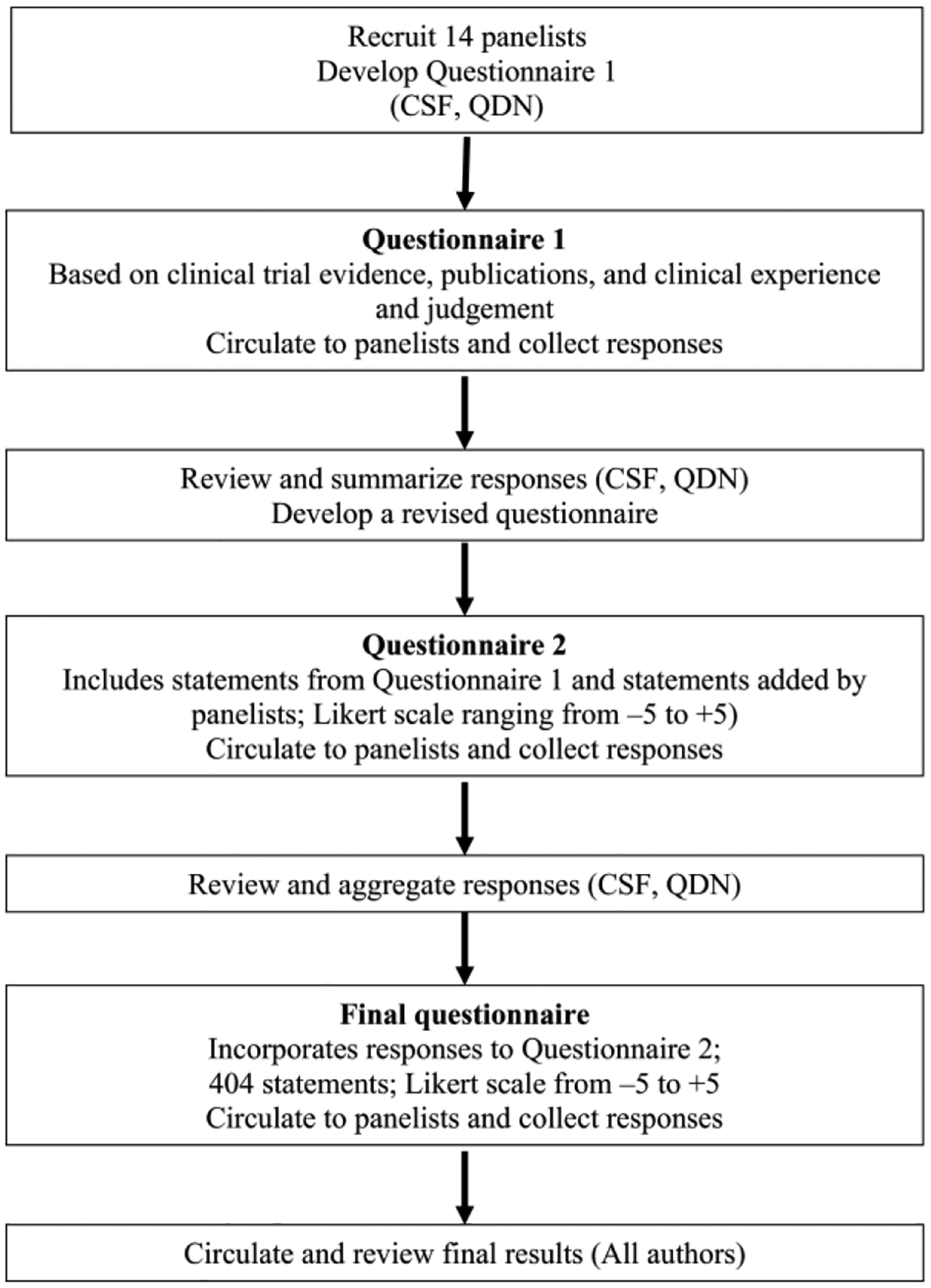
The Delphi process used in the study. CSF = C. Stephen Foster; QDN = Quan Dong Nguyen.
The first questionnaire was developed by CSF and QDN based on their clinical experience and judgment, general approach to uveitis management, clinical trial evidence, current guidelines, and other relevant publications on uveitis, and consisted primarily of open-ended questions intended to gather information on current practices for diagnosis and treatment of uveitis, the role of RCI, and strategies for managing RCI-related adverse events. Panellists’ opinions and practices were converted to definite statements, consolidated as appropriate, and included in Questionnaire 2, along with additional open response questions as needed for clarification.
Panellists were asked to rate their agreement with each statement on an 11-point Likert scale from −5 (strongly disagree) to +5 (strongly agree).
Consensus was defined as shown in Fig. 2. A mean Likert score ≥ 2.5 with a standard deviation that did not cross zero was a consensus in favour of an outcome or question. A mean Likert score ≤–2.5 with a standard deviation that did not cross zero was a consensus against the question or outcome. Other scores indicated no consensus. Results of Likert scale questions are reported as mean ± standard deviation.
Fig. 2.
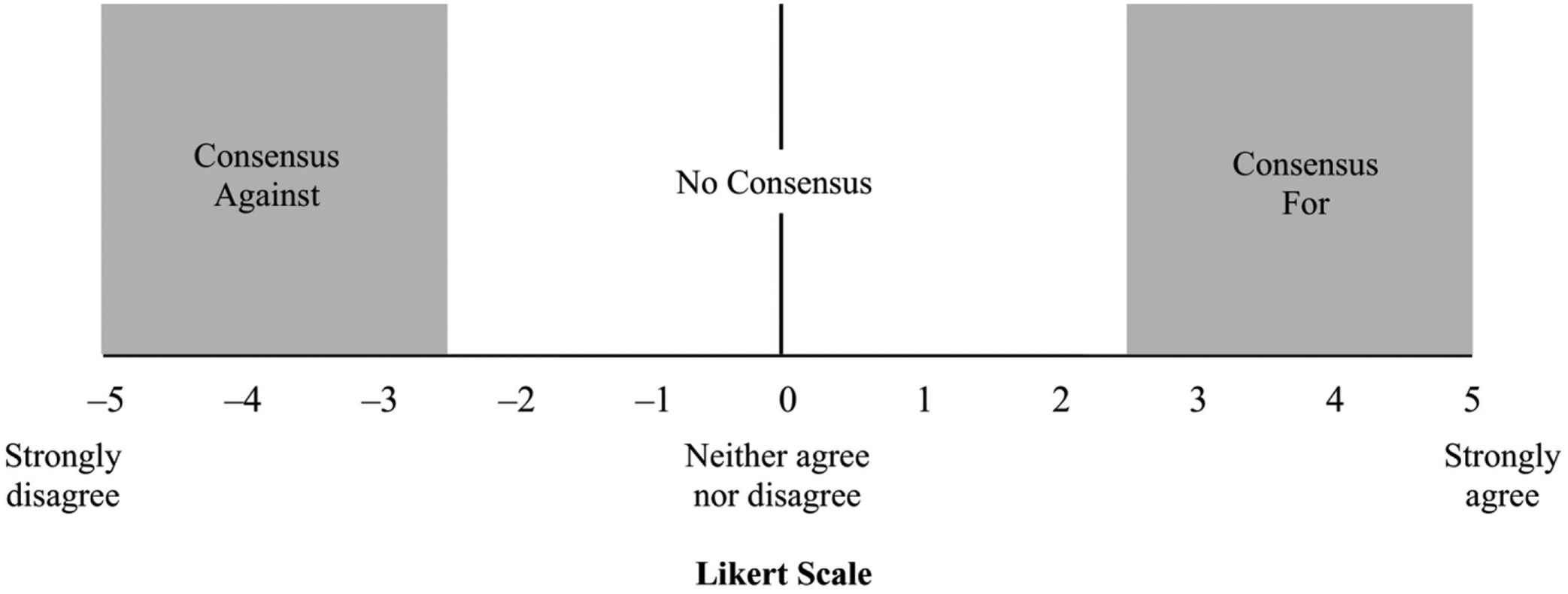
The Likert scale and definitions of consensus used throughout the Delphi process.
The final questionnaire contained the same statements and questions as Questionnaire 2, with additional statements where needed for clarification, and was circulated with a summary of the panellist’s own results to questionnaire 2 as well as the panel’s anonymized and aggregated results (mean and standard deviation [SD] of Likert scale scores). This information was provided to promote consensus by making panellists aware of the group’s opinions and allowing them the opportunity to validate or modify their responses accordingly.
As essential components of the Delphi methodology, the panellists’ anonymity was ensured throughout the study and all opinions were weighed equally, thereby minimizing risk for confirmation bias.
Results
The Delphi panel consisted of 14 physicians in active practice in the United States, managing, in parts, patients with uveitis and ocular inflammatory diseases (Table 1). All panel members participated actively in the Delphi process as defined in the Methods section. One panellist withdrew as an author. The final questionnaire comprised six major sections and included 397 Likert scale statements for which consensus was evaluated. Consensus was achieved on 208 (52.4%) statements (Table S1).
Table 1.
Characteristics of the 13 Delphi panellists
| Characteristic | Panellists (N = 13) |
|---|---|
| Specialty (n, %)* | |
| Ophthalmology – general | 7 (64%) |
| Uveitis | 9 (69%) |
| Retina | 6 (46%) |
| Immunology | 3 (23%) |
| Cornea/external diseases | 5 (38%) |
| Female gender (n, %) | 2 (15%) |
| Years in practice (median, range) | 20 (6–50) |
| Practice type (n)** | |
| Academic/University medical centre | 5 |
| Group private practice (5+ physicians) | 5 |
| Group private practice (1–4 physicians) | 3 |
| Solo private practice | 2 |
| Multi-state PE group | 1 |
| Experience treating non-infectious uveitis (median, range) | 20 (6–40) |
| Patients with non-infectious uveitis treated per year (n) | |
| <100 | 3 |
| 100–199 | 2 |
| 200–349 | 1 |
| 500–749 | 1 |
| 750–999 | 1 |
| 1000+ | 5 |
Some panellists reported multiple specialties.
Some panellists reported working in multiple practices.
Diagnosis and testing
The Delphi panel reached consensus that ophthalmologic examinations essential for diagnosis of NIU include: slit lamp examination, measurement of intraocular pressure (IOP), visual acuity testing, funduscopic examination, and spectral-domain optical coherence tomography (OCT). Fluorescein angiography (FA) should be used with OCT in selected cases. Appropriate laboratory evaluations are also essential, although consensus was not reached on the use of polymerase chain reaction (PCR), flow cytometry, or anterior chamber sampling. Panellists also reached consensus in support of a differential-based diagnostic system and an evidence-based approach. Results are presented in the supplementary materials (Table S1 and Fig. S1).
Goals of treatment and the decision to treat
The Delphi panel identified the consensus goal of therapy for anterior NIU as to maintain vision and decrease risk of cataracts and glaucoma. For posterior NIU and pan NIU, the usual goal is immunosuppression to avoid permanent retina damage and loss of vision. Results are presented in the supplementary materials (Table S1 and Fig. S2).
The panel reached consensus that factors important in the decision to initiate treatment for uveitis include: severity, duration, location, and type of uveitis, unilateral/bilateral involvement, cystoid macular oedema, elevated IOP, presence of systemic manifestations, history of flares, and sight-threatening disease.
Corticosteroids
The panel reached consensus that oral corticosteroid therapy should typically consist of prednisone at a starting dose of 1 mg/kg/day. The starting dose should be increased for patients with severe symptoms, progressive disease, or extraocular involvement, and decreased for patients with hypertension and/or brittle diabetes. Results are presented in the supplementary materials (Table S1 and Fig. S3).
Steroid-related toxicities should prompt a frank conversation with the patient and evaluation of possible toxicity at each visit. In such cases, the panel considered rapid tapering, tapering while adding immunomodulatory therapy, and removing the steroid or switching to a weaker steroid, acceptable strategies, while hypertension, worsening of glucose control, and other unacceptable side effects should result in a prompt reduction or discontinuation of oral steroid therapy. Results are presented in the supplementary materials (Table S1 and Fig. S4).
Steroid-sparing therapy should be initiated in cases of extraocular disease, steroid failure, steroid toxicity, when there is a high risk for steroid-related adverse events, anticipated long-term use, and if long-term, low-dose maintenance steroid therapy does not control the disease.
Steroid dose should be tapered in patients with stable or improved uveitis, starting after 2–4 weeks of therapy (Fig. S7). Complete weaning should be considered for patients in remission and those clinically quiescent or stable on steroid-sparing therapy. Results are presented in the supplementary materials (Table S1 and Fig. S5).
Immunomodulatory therapy
Non-biologic immunomodulators should be considered for steroid intolerance, unsatisfactory response to maximal topical steroid therapy, relapse following steroid taper, and for conditions known to require long-term therapy (such as birdshot retinochoroidopathy). Initial therapy should be methotrexate (MTX) or mycophenolate mofetil (MMF), depending on disease severity, type of disease, previous medications, the patient’s systemic history, other organ involvement, and the medication’s efficacy and adverse effect profile. Concomitant therapy could include corticosteroids and biologics. Folic acid should be prescribed with MTX, to be taken on non-MTX days. Non-biologic immunomodulators should be discontinued if ineffective, cause toxicity, fail to achieve steroid-free remission within 2–4 months, are precluded by other therapies, or in cases of patient preference. Results are presented in the supplementary materials (Table S1 and Fig. S6).
Biologic immunomodulators should be considered for patients with severe or progressive disease and/or in cases of toxicity or lack of efficacy with non-biologic immunomodulators, steroids, or a combination of the two. Unless contraindicated, a tumour necrosis factor (TNF) inhibitor, typically adalimumab (loading dose of 80 mg; maintenance dose of 40 mg every other week), should be a considered as first-line biologic. Rituximab should be used to treat anti-neutrophil cytoplasmic antibody (ANCA)-mediated vasculitides. Discontinuation of biologic therapy should be considered with development of toxicity, failure to stabilize the disease, or if the disease has been stable for at least 1–3 years. Results are presented in the supplementary materials (Table S1 and Fig. S7).
Role of RCI
Consensus statements on the role of RCI in managing uveitis are summarized in Fig. 3. Panellists reached consensus that the principal mechanism of action for RCI is melanocortin receptor-mediated immunomodulation, although the lack of side effects when RCI is given as monotherapy without concomitant prednisone supports the hypothesis that other pathways may also be involved (3.00 ± 1.78). More animal studies are needed to show whether and how these other pathways affect inflammation (3.77 ± 1.83).
Fig. 3.
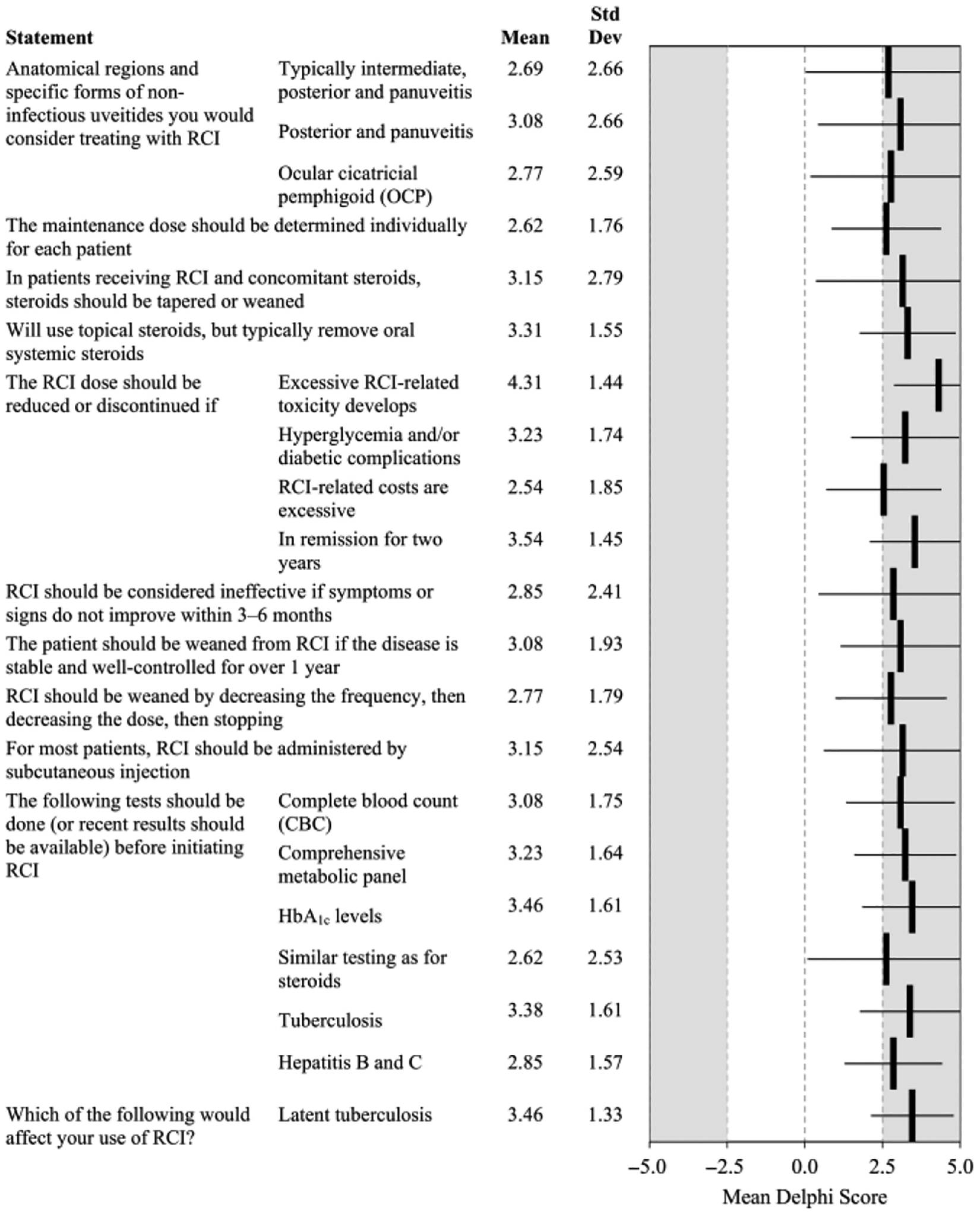
Statements on the use of RCI that reached consensus in Delphi Questionnaire 3. CBC = complete blood count; HbA1c = haemoglobin A1c; OCP = ocular cicatricial pemphigoid; RCI = repository corticotropin injection.
Panellists cited a wide variety of populations and settings in which they would use RCI in combination with other agents, ranging from none to all forms of uveitis. Detailed comments are presented in Table S1. Panellists achieved consensus to consider RCI in posterior and intermediate panuveitis (3.08 ± 2.66 and 2.69 ± 2.66, respectively), and ocular cicatricial pemphigoid (2.77 ± 2.59).
In 32 ± 42% of patients started on RCI by the panellists, the reason given was intolerance to other therapies (steroids, non-biologics, or biologics). Panellists used RCI as second-line (20 ± 24%), third-line (57 ± 59%), fourth-line (8 ± 13%), or other lines of therapy (15 ± 28%) (Fig. 4).
Fig. 4.
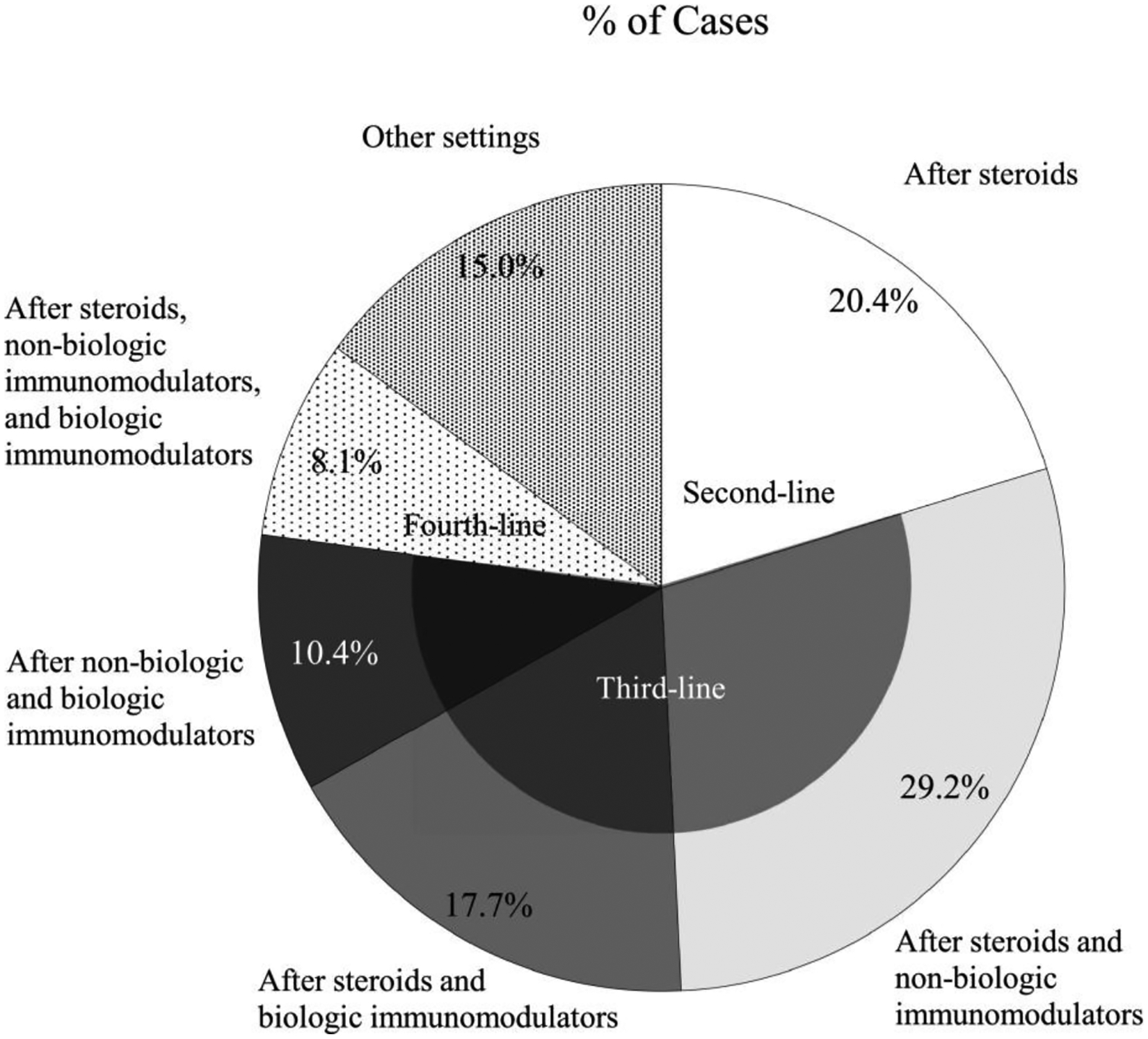
Settings in which panellists have used RCI to treat uveitis. RCI = repository corticotropin injection.
Maintenance RCI dose should be determined individually for each patient (2.62 ± 1.76). For most patients, RCI should be administered by subcutaneous injection (3.15 ± 2.54). Before initiating RCI, evaluation should be similar to steroids (2.62 ± 2.53), with results available for complete blood count (3.08 ± 1.75), comprehensive metabolic panel (3.23 ± 1.64), HbA1c levels (3.46 ± 1.61), tuberculosis (3.38 ± 1.61), and hepatitis B and C (2.85 ± 1.57). Panellists reached consensus that latent tuberculosis would affect their use of RCI (3.46 ± 1.33).
During use of RCI with concomitant medications, monitoring should be according to the standard of care for steroid therapy with appropriate laboratory and AE management. Immunosuppressants may be continued, but dose should be tapered as tolerated. Concomitant steroids should be tapered or weaned (3.15 ± 2.79), including oral steroids (3.31 ± 1.55).
Patients receiving RCI who are hospitalized should receive steroids at a stress dose (i.e. exogenous steroids administered at a sufficient dose to reduce the risk for adrenal insufficiency) (2.62 ± 2.26) with continued RCI therapy unless the hospitalization is for infection (2.69 ± 1.93), in particular life-threatening infection (3.85 ± 1.63) or sepsis (3.23 ± 2.39), or if the cause of the hospitalization is probably related to RCI (3.31 ± 1.75).
Repository corticotropin injection (RCI) should be down-titrated or discontinued if AEs are problematic and other interventions to manage the AEs fail, and patients should be weaned from RCI if disease is stable and well controlled for over 1 year (3.08 ± 1.93). Repository corticotropin injection (RCI) should be weaned by decreasing the frequency, then decreasing the dose, then stopping (2.77 ± 1.79). Repository corticotropin injection (RCI) dose should be reduced or RCI discontinued in cases of excessive RCI-related toxicity (4.31 ± 1.44), hyperglycaemia and/or diabetic complications (3.23 ± 1.74), remission for at least two years (3.54 ± 1.45), or if RCI-related costs are excessive (2.54 ± 1.85). Repository corticotropin injection (RCI) should be considered ineffective if symptoms or signs do not improve within 3–6 months (2.85 ± 2.41).
Management of adverse events during therapy with RCI
Panellists considered a variety of adverse events potentially associated with RCI therapy including oedema, anxiety/depression, infection, increased appetite/weight gain, glucose intolerance/worsening in glucose control, systemic hypertension, skin darkening and other skin-related adverse events, localized injection site pain, and insomnia (Table 2). For systemic oedema, panellists recommended evaluation of potential causes (4.31 ± 0.95) and referral to the patient’s primary care physician (3.85 ± 1.46). For ocular oedema, panellists recommended consideration of dexamethasone intravitreal implants (2.77 ± 1.36) or a sub-Tenon’s steroid injection (3.15 ± 1.21). For anxiety and/or depression, panellists recommended standard treatment, possibly with a referral to primary care psychiatry (3.92 ± 1.04). For infection, panellists recommended appropriate antimicrobial therapy (first-line: 4.31 ± 1.38; second-line: 3.08 ± 1.55). Behavioural intervention (2.62 ± 1.66), dietary counselling (3.00 ± 1.53), and exercise (3.31 ± 1.84) were recommended for increased appetite or weight gain. Glucose intolerance or worsening in glucose control should be managed as for oral steroids (3.08 ± 1.32). Management of hypertension should include education on sodium and fluids (2.92 ± 1.75), use of antihypertensive medications (first-line: 3.23 ± 1.59; second-line: 2.69 ± 1.6), and referral to cardiology (2.92 ± 1.61). Darkening of the skin should be managed by discussion with the patient and a referral to a dermatologist (3.46 ± 1.33). Recommendations for other skin-related adverse events (AEs) included rotation of the injection site (3.38 ± 1.56) and referral to a dermatologist (3.15 ± 1.21). Localized injection site pain can be managed by cooling the skin with an ice pack (4.15 ± 0.9), rotating the injection sites or using a slower injection rate (4.31 ± 0.75), and educating the patient on injection technique (4.31 ± 0.75). Ibuprofen may also be useful (2.62 ± 1.39). Insomnia should be managed with good sleep hygiene (3.69 ± 1.44). Recommendations for RCI dose adjustments are summarized in Table 2. The panel reached consensus that, for most AEs, RCI dose should be down-titrated or discontinued if other interventions fail or to manage severe AEs. However, panellists did not reach consensus on RCI down-titration for localized injection site pain or skin-related AEs other than skin darkening.
Table 2.
Consensus recommendations for RCI dose adjustment or discontinuation for management of selected adverse events (AEs) in patients receiving treatment with RCI for uveitis
| Adverse event | Dose adjustment | |||
|---|---|---|---|---|
| Down titrate if other interventions fail | Down titrate concomitantly with other interventions for severe AEs | Discontinue if other interventions fail | Discontinue for severe, significant AEs | |
| Oedema | 2.92 ± 1.61 | 3.62 ± 1.56 | 2.92 ± 1.85 | 4.23 ± 1.42 |
| Anxiety/Depression | 3.15 ± 1.46 | 3.62 ± 1.50 | 3.31 ± 1.70 | 4.15 ± 1.52 |
| Infection | 3.15 ± 1.82 | 3.62 ± 1.71 | 3.31 ± 1.97 | 4.00 ± 1.63 |
| Increased Appetite/Weight Gain | 2.85 ± 1.72 | 3.23 ± 1.92 | 3.15 ± 1.99 | 4.08 ± 1.61 |
| Glucose Intolerance/Worsening in Glucose Control | 3.31 ± 1.44 | 3.92 ± 1.44 | 3.54 ± 1.61 | 3.92 ± 1.75 |
| Hypertension | 2.85 ± 1.82 | 3.46 ± 1.94 | 3.00 ± 2.00 | 4.08 ± 1.50 |
| Darkening of the Skin | 2.54 ± 2.22 | 2.85 ± 2.34 | 2.62 ± 2.33 | 3.46 ± 2.07 |
| Other Skin-related AEs | 2.46 ± 2.18 | 3.15 ± 2.34 | 2.62 ± 2.43 | 3.54 ± 2.03 |
| Localized Injection Site Pain | 2.00 ± 2.83 | 2.38 ± 3.01 | 2.62 ± 2.14 | 3.38 ± 2.06 |
| Insomnia | 2.92 ± 1.71 | 3.15 ± 1.91 | 2.77 ± 1.88 | 3.77 ± 1.69 |
Recommendations that reached consensus are bold. Values are the mean ± standard deviation of the Likert scale scores (range: −5 to +5). Consensus was defined as a mean value ≥ 2.5 (for) or ≤−2.5 (against) with a standard deviation which did not cross zero.
AE = adverse event.
Discussion
This Delphi panel was employed to develop consensus recommendations for the diagnosis and treatment of uveitis, with a particular focus on the role of RCI in treating non-infectious uveitis.
Uveitis may result from numerous aetiologies, including infection, autoimmune disorders, neoplasia, and a variety of masquerade conditions. As a result, the diagnosis and evaluation of uveitis are challenging and require a broad array of evaluations. The panellists’ consensus on diagnosis emphasizes the need for a comprehensive approach to diagnosis, with ophthalmologic assessments (slit lamp, IOP measurement, visual acuity testing, funduscopic examination, and OCT) and laboratory evaluations. While the panellists did not reach consensus on the use of FA (2.23± 1.79), the lead authors feel strongly that it is a critical part of the evaluation of posterior uveitis, intermediate uveitis, or panuveitis, in addition to cases with anterior uveitis and decreased vision, because it can detect subtle macular oedema, optic disc inflammation, or retinal vasculitis. The lead authors feel that this lack of consensus reflects the increasing use of OCT and possibly OCT angiography (OCTA), which has displaced FA for selected indications in some practices. However, currently available data on OCT and OCTA are not sufficient to demonstrate that they can provide similar information as FA for peripheral retinal vascular diseases. For instance, OCTA cannot be used for peripheral retinal vasculitis.
The current literature on the treatment of uveitis generally recommends corticosteroids as the initial therapy, with additional immunomodulatory therapy added if corticosteroids are not sufficiently effective, cause significant AEs, or if the physician expects that long-duration therapy will be required (Foster et al. 2013; Pleyer et al. 2014; Foster et al. 2016). Our Delphi recommendations agree with this approach. However, the Delphi panel did not provide detailed recommendations on the selection and use of systemic corticosteroids and immunomodulatory agents. Notably, the Delphi panel did not reach consensus on the need for steroid-sparing therapy for steroid doses >5 mg/day. The lack of consensus may reflect disagreement on the appropriate threshold for steroid-sparing therapy; some panellists may use thresholds of 7.5 mg/day or 10 mg/day.
Appropriate use of these agents requires careful consideration of their potential systemic adverse effects, the patient’s individual characteristics, and any concomitant diseases and medications, so developing detailed recommendations is difficult. Given that systemic immunomodulatory therapies are usually managed by rheumatologists or other specialists familiar with systemic chemotherapies, further efforts to develop consensus recommendations on these topics should include panellists from the relevant collaborative specialties in addition to ophthalmologists.
There is a clear need for clinical information on RCI given its receipt of Food and Drug Administration (FDA) approval for this indication in 1952 and the marked lack of recent clinical evidence on its use. To our knowledge, recent published data on RCI in uveitis are limited to one case report in a 33-year-old Caucasian male who was initially treated with oral corticosteroids and tocilizumab in a clinical trial but relapsed 6 months after completion of the study. He was unable to resume therapy with tocilizumab due to denial of insurance coverage and initiated treatment with RCI. The clinical response to RCI was satisfactory and RCI therapy was ongoing when the case report was published (Agarwal et al. 2016).
The Delphi panel reached consensus that RCI should be considered for posterior uveitis, intermediate uveitis, and panuveitis, but not anterior uveitis. With the exception of ocular cicatricial pemphigoid, specific conditions for which RCI should be considered were not identified. Consistent with the general lack of clinical experience with RCI in uveitis, there was no consensus on dosing beyond the need to individualize the dose for each patient. Panellists reached consensus that RCI should be administered by subcutaneous injection. The RCI dose should be reduced or discontinued for toxicity, lack of response, or excessive costs, and the patient should be weaned from RCI if the disease is stable and well controlled for at least 1 year. Weaning should start with decreasing the dosing frequency and then decreasing the dose before stopping. Most AEs can be managed with appropriate pharmacological and non-pharmacological interventions. If these fail or the AE is severe, the RCI dose can be reduced or discontinued. The panellists’ consensus that the mechanism of action for RCI involves melanocortin receptor-mediated immunomodulation may have important implications for the therapeutic role of RCI. Repository corticotropin injection (RCI) is believed to affect steroid-independent pathways that regulate autoimmunity (Catania et al. 2010). The effects of RCI are not limited to stimulation of steroid production, suggesting that RCI may be valuable as a steroid-sparing option for the management of uveitis. Studies investigating this possibility are ongoing (NCT02725177 2016; NCT02931175 2018; NCT03473964 2018).
The Delphi method has been broadly accepted as a strategy for developing consensus recommendations in the absence of clear clinical evidence because the Delphi process is systematic, gives all panellists’ opinions equal weight, provides anonymity to promote free exchange of opinions and ideas, and prevents domination of the results by any one panellist. The use of email to distribute questionnaires and gather the panellists’ responses was convenient and helped maintain anonymity throughout the Delphi process.
The limitations of the Delphi method must, however, be acknowledged. In our study, panel selection and the development of the initial questionnaire was managed by the lead authors. Although the lead authors attempted to select a wide range of panellists and maintain neutrality developing the initial questionnaire, these steps may have introduced bias in part into the process (Hsu & Sandford 2007; Phillips et al. 2013). All panellists were from and their practices were located in the US, raising the likelihood of biasing the resulting recommendations towards US practice. Panellist selection relied on self-report of their experience managing non-infectious uveitis, and the use of RCI for this purpose. None of the panel members were specialists in the management of the systemic effects of immunomodulatory medications which may have limited the value of the panel’s consensus on related topics.
The Delphi process only produces a consensus of opinion which could be refuted by evidence from clinical studies (Powell 2003; Phillips et al. 2014; Pleyer et al. 2014). There is a lack of generally accepted criteria to define consensus in Delphi studies, although this is probably unavoidable given the wide variety of topics addressed with this approach. (Powell 2003; Phillips et al. 2013; Phillips et al. 2014). Although anonymity is critical to the process, it may empower panellists to feel less accountable for their responses, or to provide responses that are not based on full, careful consideration (Powell 2003). This could be a particular issue for questionnaires that require a substantial time commitment to complete.
The modified Delphi process produced a consensus that RCI may be an appropriate treatment option for patients with intermediate, posterior, or panuveitis for whom steroids and/or non-biologic or biologic immunomodulatory therapies have been ineffective or cause excessive toxicity. The panel also provided practical guidance on real-world issues in managing AEs in patients during treatment of uveitis with RCI. Additional studies of RCI in this indication are clearly needed to improve our understanding of appropriate roles for RCI and strategies for dosing, titration and AE management.
Supplementary Material
Acknowledgments
Dr. Nguyen has received funding from the Research to Prevent Blindness and NIH grant P30-EY026877 and serves on the scientific advisory boards for EyePoint, Genentech, Gilead, Regeneron, and Santen, among others. Dr. Nguyen also chairs the study steering committees for the SAVE, SAVE-2, STOP-UVEITIS, VISUAL, ACTHAR and SAKURA studies. Dr. Anesi serves as a consultant for Takeda (formerly Shire), Allakos, Eyepoint Pharmaceuticals and Santen. Dr. Anesi also is a speaker for AbbVie, Eyepoint Pharmaceuticals and Mallinckrodt. Dr. Chexal serves as a consultant for Allergan. Dr. Chu serves as a consultant for Mallinckrodt Pharmaceuticals, Abb-Vie, Aldeyra Therapeutics, Allakos Inc., and Santen Pharmaceuticals. Dr. Dayani serves on the scientific advisory boards for Alimera, Aldeyra and Abbvie. Dr. Leng has received research funding from Topcon, Genentech and the Research to Prevent Blindness and NIH grant P30-EY026877, and serves as a consultant for Allergan. Dr. Meleth serves as a consultant for Allergan and EyePoint and also serves as a speaker for EyePoint. Dr. Sallam has no financial disclosures. Dr. Sheppard reports academic clinical trial work in the field of uveitis, and consultation or lecture work with Mallinckrodt, Abbvie, Aldeyra, Allergan, Alcon, Novartis, Bausch & Lomb, Sun, EyePoint, Santen and Kala. None of the research involved ownership interests or remuneration based upon outcomes of trials. Dr. Silverstein has served as a lecturer for Mallinckrodt. Dr. Toyos has served as a speaker and consultant for and received research funding from Bausch & Lomb, Takeda, Iridex, Sun Pharmaceuticals, Zeiss, Mallinckrodt, and MIXTOLasering; received research funding from Oculos, Magellan, Ocular Tech, ReVitalLi, Digi-sight, Opternative, Gilead Sciences and Kala; served as a speaker and received research funding from Lumenis; and consulted with Eyevance. Dr. Wang has served as a speaker for Abbvie, Mallinckrodt and EyePoint. Dr. Foster has no financial disclosures.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Treatment of non-infectious uveitis with RCI: Delphi Questionnaire 3 results.
Figure S1. Statements on diagnosis and testing of uveitis evaluated in Delphi Questionnaire 3.
Figure S2. Statements on the goals of treatment and decision to treat evaluated in Delphi Questionnaire 3.
Figure S3. Statements on the use of systemic corticosteroids that reached consensus in Delphi Questionnaire 3.
Figure S4. Statements on systemic corticosteroid dose adjustments for toxicities or lack of efficacy/response that reached consensus in Delphi Questionnaire 3.
Figure S5. Statements on corticosteroid weaning and discontinuation that reached consensus in Delphi Questionnaire 3.
Figure S6. Selected statements on the use of non-biologic immunomodulators that reached consensus in Delphi Questionnaire 3.
Figure S7. Selected statements on the use of biologic immunomodulators that reached consensus in Delphi Questionnaire 3.
References
- AbbVie (2018): HUMIRA® (adalimumab) [Prescribing Information]. North Chicago, IL: AbbVie Inc. [Google Scholar]
- Agarwal A, Hassan M, Sepah YJ, Do DV & Nguyen QD (2016): Subcutaneous repository corticotropin gel for non-infectious panuveitis: Reappraisal of an old pharmacologic agent. Am J Ophthalmol Case Rep 4: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K & Auer H (2012): Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR (2010): A look at autoimmunity and inflammation in the eye. J Clin Invest 120: 3073–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania A, Lonati C, Sordi A, Carlin A, Leonardi P & Gatti S (2010): The melanocortin system in control of inflammation. ScientificWorldJournal 10: 1840–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq AL, van de Ven AH & Gustafson DH (1975): Group techniques for program planning. Glenview, IL: Scott, Foresman, and Co. [Google Scholar]
- Foster CS, Kothari S, Anesi SD, Vitale AT, Chu D, Metzinger JL & Ceron O (2016): The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol 61: 1–17. [DOI] [PubMed] [Google Scholar]
- Foster CS, Vitale AT & Jakobiec FA (2013): Diagnosis and Treatment of Uveitis. : New Delhi, IndiaJaypree Brothers Medical Publishers. [Google Scholar]
- Gonzalez MM, Solano MM, Porco TC, Oldenburg CE, Acharya NR, Lin SC & Chan MF (2018): Epidemiology of uveitis in a US population-based study. J Ophthalmic Inflamm Infect 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz DC & Wong IG (2004): Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 111: 491–500.discussion 500. [DOI] [PubMed] [Google Scholar]
- Gupta R & Murray PI (2006): Chronic non-infectious uveitis in the elderly: epidemiology, pathophysiology and management. Drugs Aging 23: 535–558. [DOI] [PubMed] [Google Scholar]
- Haines S, Baker T & Donaldson M (2013): Development of a physical performance assessment checklist for athletes who sustained a lower extremity injury in preparation for return to sport: a Delphi study. Int J Sports Phys Ther 8: 44–53. [PMC free article] [PubMed] [Google Scholar]
- Hasson F, Keeney S & McKenna H (2000): Research guidelines for the Delphi survey technique. J Adv Nurs 32: 1008–1015. [PubMed] [Google Scholar]
- Hsu CC & Sandford BA (2007): The Delphi technique: making sense of consensus. Pract Assess Res Eval 12: 1–8. [Google Scholar]
- Jabs DA, Rosenbaum JT, Foster CS et al. (2000): Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 130: 492–513. [DOI] [PubMed] [Google Scholar]
- Mallinckrodt (2018): H.P. Acthar® Gel (repository corticotropin injection). [Prescribing Information]. Bedminster, NJ: Mallinckrodt Pharmaceuticals. [Google Scholar]
- Mansell G, Shapley M, van der Windt D, Sanders T & Little P (2014): Critical items for assessing risk of lung and colorectal cancer in primary care: a Delphi study. Br J Gen Pract 64: e509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meyrick J (2003): The Delphi method and health research. Health Educ 103: 7–16. [Google Scholar]
- NCT02725177 (2016): Ocular sarcoidosis open label trial of ACTHAR gel. Clinicaltrials.gov.
- NCT02931175 (2018): ACTH as A Re-emerging theRapy for Uveitis (The ACTHAR Study) (ACTHAR). ClinicalTrials.gov
- NCT03066869 (2017): Efficacy and safety of H.P. ACTHAR GEL in adults with retinal vasculitis Clinicaltrials.gov.
- NCT03473964 (2018): (ACTH) for the Treatment of Sarcoid Uveitis (ACTH). ClinicalTrials.gov.
- NCT03727776 (2018): Adrenocorticotropic Hormone (ACTH) for Post-op Inflammation in Proliferative Vitreoretinopathy (PVR). Clinicaltrials.gov.
- Nguyen QD, Hatef E, Kayen B, Macahilig CP, Ibrahim M, Wang J, Shaikh O & Bodaghi B (2011): A cross-sectional study of the current treatment patterns in noninfectious uveitis among specialists in the United States. Ophthalmology 118: 184–190. [DOI] [PubMed] [Google Scholar]
- Opremcek EM (1995): A Clinical Manual for Ocular Infalmmation. New York, NY: Springer-Verlag. [Google Scholar]
- Phillips AC, Lewis LK, McEvoy MP et al. (2013): Protocol for development of the guideline for reporting evidence based practice educational interventions and teaching (GREET) statement. BMC Med Educ 13: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Lewis LK, McEvoy MP et al. (2014): A Delphi survey to determine how educational interventions for evidence-based practice should be reported: stage 2 of the development of a reporting guideline. BMC Med Educ 14: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersma S, de Vries M & van den Akker-van Marle ME (2014): Domains of quality of life: results of a three-stage Delphi consensus procedure among patients, family of patients, clinicians, scientists and the general public. Qual Life Res 23: 1543–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleyer U, Alio J, Barisani-Asenbauer T, Le Hoang P & Rao NA (2014): Immune Modulation and Anti-Inflammatory Therapy in Ocular Disorders: IOIS Guidelines. Berlin Heidelberg: Springer Verlag. [Google Scholar]
- Powell C (2003): The Delphi technique: myths and realities. J Adv Nurs 41: 376–382. [DOI] [PubMed] [Google Scholar]
- Rahaghi FF, Feldman JP, Allen RP et al. (2017): Recommendations for the use of oral treprostinil in clinical practice: a Delphi consensus project pulmonary circulation. Pulmon Circ 7: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Calonge M, Pedroza-Seres M, Akova YA, Messmer EM, D’Amico DJ & Foster CS (1996): Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol 114: 593–599. [DOI] [PubMed] [Google Scholar]
- Schutt AC, Bullington WM & Judson MA (2010): Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med 104: 717–723. [DOI] [PubMed] [Google Scholar]
- Smith RE & Nozik RA (2003): Uveitis. A Clinical Approach to Diagnosis and Management Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- Suttorp-Schulten MS & Rothova A (1996): The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol 80: 844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW & Lee DJ (2011): The alpha-melanocyte stimulating hormone induces conversion of effector T cells into treg cells. J Transplant 2011: 246856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J & Ganguli A (2016): Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol 134: 1237–1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


