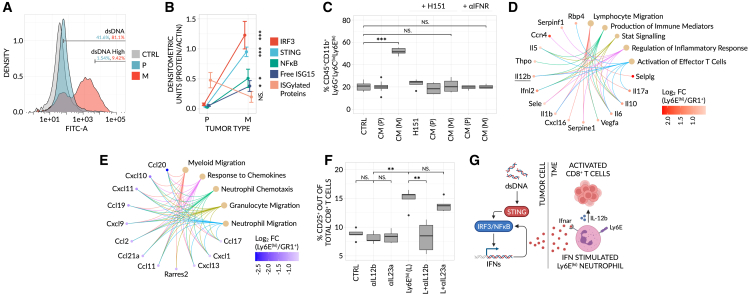
Figure 5.
Tumor-intrinsic STING activity induces the Ly6E(hi) phenotype and in-turn supports activation of effector T cells
(A) Density plots of dsDNA levels in cultured 4T1P and 4T1M cell-lines, as determined by α-dsDNA staining and flow cytometry. dsDNA levels were quantified relative to an unstained, IgG2a isotype control (CTRL) (n = 5 biological repeats/group).
(B) Densitometry quantification of western blots (see Figure S5A) for STING-pathway related proteins in 4T1P and 4T1M tumor lysates (n = 3–4 biological repeats/group). Each protein was normalized relative to an actin control.
(C) Isolated GR1+ cells were cultured in vitro with conditioned media generated from 4T1P (P) or 4T1M (M) tumors in the presence or absence of the STING-inhibitor H151 or αIFNR-α/γ, and the frequencies of Ly6E(hi) neutrophils were determined by flow cytometry (n = 6 biological repeats/group). CTRL = GR1+ cells only.
(D and E) Conditioned media was generated from GR1+ cells or IFNαγ-induced Ly6E(hi) neutrophils, and subsequently assayed on a cytokine array (n= 3 mice pooled/group). Hyper-geometric, over-representation tests and the Gene Ontology (GO) database were used to determine enriched pathways for Ly6E(hi) neutrophils (D); and GR1+ cells (E). Only differentially expressed proteins with a log2FC > 0.35 were included and only significant pathways (FDR < 0.01) are shown.
(F) Isolated CD8+ T cells were cultured in vitro with α-IL-12b or α-IL23a neutralizing antibodies, with or without conditioned media from IFNα/γ-induced Ly6E(hi) neutrophils (L), and the levels of activated CD25+CD8+ T cells were determined by flow cytometry (n = 5 mice/group). CTRL = CD8+ T cells only. In (B, C, and F), significance was assessed by means of a one-way ANOVA and Tukey’s post-hoc HSD test (NS, p > 0.01; ∗, p < 0.01; ∗∗, p < 0.001; ∗∗∗, p < 0.0001).
(G) Schematic of the proposed mechanism. Tumor-intrinsic STING activity, as induced by cytosolic dsDNA as a result of hypoxia, genomic instability and/or cell stress, transcriptionally activates an IFN response. Tumor-secreted IFNα, for example, subsequently binds to Ifnar-expressing Neutrophils in the TME, inducing the Ly6E(hi) phenotype and in-turn activation and proliferation of CD8+ T cells through IL-12b. Collectively, this supports immunotherapy response and anti-tumor activity. It is important to note that this mechanism is STING-specific, but that Type II IFNs (e.g., IFNγ)—derived from other sources or mechanisms—are also able to elicit equivalent effects, as shown in our work.