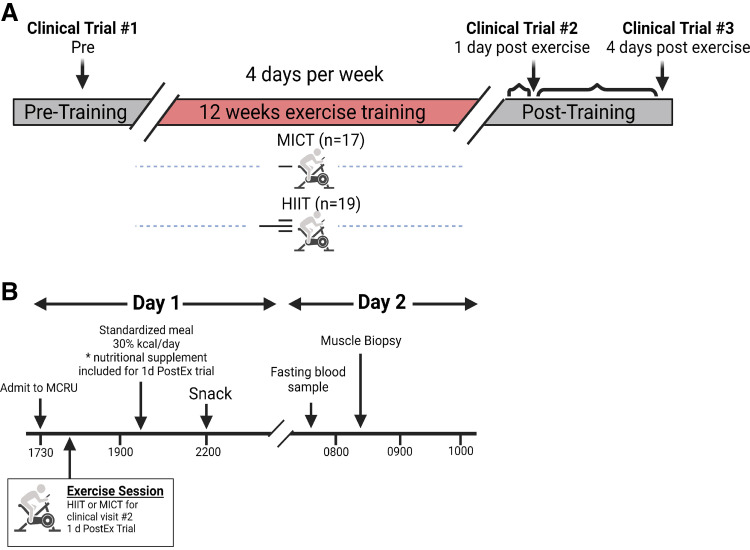
Figure 1.
Overall study design and clinical trial schematic. A: overall study design. A total of 48 sessions were completed during the 12-wk training intervention (4 days/wk). Clinical trials were completed once before training (Clinical Trial #1: Pre), 1 day after the last exercise session after the training period (Clinical Trial #2: 1d PostEx), and 4 days after the last exercise session (Clinical Trial #3: 4d PostEx). B: clinical trial schematic. Participants arrived to the Clinical Research Unit at 1730 h the day before metabolic testing and were provided a standardized meal. For Clinical Trial #2 only, the respective exercise session was completed at ∼1800 h. Muscle biopsies were obtained at ∼0815 h. *Nutritional supplement kcals were estimated to match energy expenditure from the previous exercise session. Created with BioRender.com.