Keywords: glucose homeostasis, nonalcoholic fatty liver disease, obesity, resistance exercise training, time-restricted feeding
Abstract
Nonalcoholic fatty liver disease (NAFLD), a condition characterized by the accumulation of fat in the liver, is estimated to be the most common liver disease worldwide. Obesity is a major risk factor and contributor, and, accordingly, weight loss can improve NAFLD. Previous studies in preclinical models of diet-induced obesity and fatty liver disease have shown the independent benefits of resistance exercise training (RT) and time-restricted feeding (TRF) in preventing weight gain and hepatic build-up of fat. Here, we tested the combined effect of TRF and RT on obesity and NAFLD in mice fed a high-fat diet. Our results showed that both TRF—8-h food access in the active phase—and RT—consisting of three weekly sessions of ladder climbing—attenuated body weight gain, improved glycemic homeostasis, and decreased the accumulation of lipids in the liver. TRF combined with RT improved the respiratory exchange rate, energy expenditure, and mitochondrial respiration in the liver. Furthermore, gene expression analysis in the liver revealed lower mRNA expression of lipogenesis and inflammation genes along with increased mRNA of fatty acid oxidation genes in the TRF + RT group. Importantly, combined TRF + RT was shown to be more efficient in preventing obesity and metabolic disorders. In conclusion, TRF and RT exert complementary actions compared with isolated interventions, with significant effects on metabolic disorders and NAFLD in mice.
NEW & NOTEWORTHY Whether time-restricted feeding (TRF) combined with resistance exercise training (RT) may be more efficient compared with these interventions alone is still unclear. We show that when combined with RT, TRF provided additional benefits, being more effective in increasing energy expenditure, preventing weight gain, and regulating glycemic homeostasis than each intervention alone. Thus, our results demonstrate that TRF and RT have complementary actions on some synergistic pathways that prevented obesity and hepatic liver accumulation.
INTRODUCTION
Obesity and its comorbidities have become major global health problems and are ranked the fifth most common cause of death worldwide (1). Obesity is a complex health problem resulting from genetic and behavioral factors (2, 3) and is aggravated by physical inactivity, increased calorie intake, and living in an obesogenic environment (4, 5). In addition, obesity is associated with several metabolic alterations (i.e., dyslipidemia, glucose intolerance, and increased blood pressure), which are risk factors for cardiovascular disease, type 2 diabetes mellitus (T2DM), and nonalcoholic fatty liver disease (NAFLD) (6). NAFLD is characterized by marked accumulation of fat in the liver. The incidence of NAFLD is associated with metabolic syndrome, having a higher frequency in patients with obesity and T2DM (7, 8). NAFLD, the most common cause of chronic liver disease, starts with steatosis but can progress to nonalcoholic steatohepatitis in which significant liver fibrosis and cirrhosis are present, ultimately leading to hepatocellular carcinoma (9).
Preventing weight gain and inducing weight loss are efficient strategies to halt liver steatosis development. This can be achieved through behavioral interventions, such as time-restricted feeding (TRF) or physical activity. Preclinical studies show that TRF can mitigate the harmful effects of obesity on hepatic metabolism. TRF, in which access to food is limited to 8–10 h during the active phase, leading to an extended period of fasting (i.e., >12 h per 24 h), can prevent high-fat diet-induced adverse effects on metabolic health (10–12). TRF has been shown to reduce body weight, glycemia, and insulinemia, improve lipid and inflammatory profiles, and enhance insulin sensitivity in experimental models (12–14).
Exercise can also mitigate liver steatosis. In the literature, preclinical studies carried out with rodents submitted to both aerobic exercise (15–17) and resistance exercise (18, 19), as well as studies of clinical trials, showed that aerobic exercise (20–25) and resistance training (26, 27) reduce hepatic fat content. However, when comparing the effects of aerobic exercise with resistance exercise, there is still no consensus on which model is more beneficial for the treatment of hepatic steatosis. Previously, we showed that three exercise protocols (aerobic, resistance, and aerobic combined with resistance) were successful in reducing the concentration of hepatic triglycerides, with the resistance exercise group in fructose-fed rats presenting the smallest reserves of hepatic triglycerides among all the exercised groups (28). On the other hand, Bacchi et al. (29) compared the effects of aerobic or resistance training on hepatic fat content in subjects with type 2 diabetes (T2DM) with NAFLD. The study demonstrated that both resistance training and aerobic training are effective in reducing hepatic fat content among patients with T2DM with NAFLD. A cohort study involving 10,500 men who engaged in a variety of physical activities during the years 1996 and 2008 demonstrated that weight training had the strongest association with the lowest increase in waist circumference when compared with other moderate-to-vigorous aerobic activities (30).
In clinical practice, dietary interventions are usually accompanied by guidelines and recommendations for engaging in physical activity. Regular physical exercise leads to beneficial physiological and clinical adaptations, such as increased cardiovascular capacity, insulin sensitivity, improved blood glucose, and blood pressure, as well as regulating body weight by reducing body fat deposits and enhancing lean mass (31). These benefits are especially crucial in preventing and treating chronic diseases, such as obesity, DM2, and cardiovascular diseases (32), and NAFLD (33–35). Although it has been established that TRF and physical exercise can curtail metabolic disorders individually, few studies have investigated their combined effect. Recently, our research group took the first step toward understanding how the TRF paradigm interacts with physical training (14). We showed that when combined with aerobic exercise training, TRF provided complementary benefits, with significant effects on body adiposity, insulin sensitivity, and hepatic metabolism, acting preventively against the development of NAFLD (14). Likewise, the study by Ezpeleta et al. (36) demonstrated that the association of alternate days of fasting with aerobic exercises reduced body weight, fat mass, and waist circumference, while increasing insulin sensitivity in obese adults with NAFLD. However, the authors demonstrated that the benefits of TRF combined with aerobic exercise were not greater compared with TRF alone (36). Here, we set out to determine the unknown effect of combining resistance exercise training (RT) with TRF with the hypothesis that these two interventions might have complementary benefits on weight control and hepatic disease in mice fed a high-fat diet.
MATERIALS AND METHODS
Animals and Ethical Procedures
All male Swiss mice (Mus musculus), aged 4 wk, were obtained from the Multidisciplinary Center for Biological Investigations in Laboratory Animal Science (CEMIB)—State University of Campinas (UNICAMP) (n = 60). The mice were allowed a 2-wk adaptation period to the vivarium of the Laboratory of Molecular Biology of Exercise (UNICAMP). At 6 wk of life, they were submitted to the different experiments of the study. The entire experiment was conducted in two cohorts, and the number of mice used in each analysis is described in the figure captions. All experiments were carried out according to the Brazilian legislation on the scientific use of animals (Law 11,794, of October 8, 2008) and were approved by the Ethics Committee on Animal Use (CEUA/Process Number 5703-1/2021) and by the Institute of Biological Sciences of UNICAMP (Campinas-SP). In addition, the experiments were aligned with the National Council for Animal Experimentation Control (CONCEA). The animals were kept in individual polyethylene cages under controlled conditions with a light-dark cycle (12/12 h), temperature (21 ± 2°C), relative humidity (45%–55%), and free access to water and standard chow or high-fat diet (37), according to the American Institute of Nutrition (AIN39-G) (38). The animals were weighed weekly, and food intake was controlled daily by monitoring the weight of the remaining food.
Experimental Group
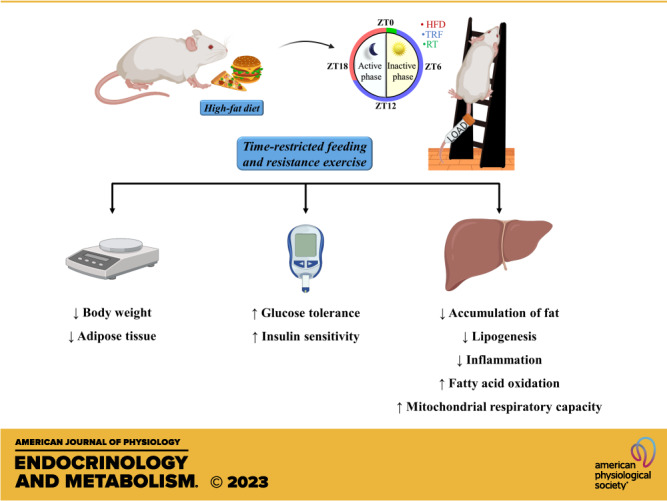
Young Swiss mice (aged 6 wk) were distributed into five experimental groups: 1) Control group (CTL): mice that received standard diet ad libitum; 2) Obese mice (OB), mice were fed with a high-fat diet ad libitum; 3) Mice submitted to time-restricted feeding (TRF), fed with a high-fat diet for 8 h followed by 16 h of food restriction; 4) Mice submitted to the resistance exercise training protocol (RT), fed with a high-fat diet ad libitum; 5) Mice submitted to TRF combined with RT (TRF + RT), fed with a high-fat diet for 8 h followed by 16 h of food restriction. The experiments were carried out over 8 wk. Physiological analyses were performed after a fasting period of 4 h. The experimental design is summarized in Fig. 1A.
Figure 1.
Experimental design. A: experimental design. The animals were distributed into five experimental groups: control group (CTL), obese mice (OB), time-restricted feeding (TRF), resistance exercise training (RT), and TRF + RT. The TRF, RT, and TRF + RT were submitted to time-restricted feeding (TRF) and/or resistance exercise training (RT) interventions for 8 wks for further analysis. B: the TRF protocol was performed during the animals’ light and dark cycles. CLAMS, Comprehensive Laboratory Animal Monitoring System; GTT, glucose tolerance test; ITT, insulin tolerance test; MVCC, maximum voluntary carrying capacity; ZT, zeitgeber time.
Time-Restricted Feeding Protocol
Mice from the TRF and TRF + RT groups were submitted to the TRF protocol as described in Fig. 1B. Zeitgeber time (ZT) 0 was designated as “lights on” time and ZT12 as “lights off” time. Access to the diet occurred 4 h after the beginning of the dark phase (ZT16) until the beginning of the light cycle (ZT0), totaling 8 h of diet access (14). Access to food was regulated by transferring the mice daily between cages with free access to food and water to cages with free access only to water. Thus, these animals had access to food for 8 h and were fasted for 16 h a day.
Description and Adaptation to the Resistance Training and Determination of Maximum Voluntary Carrying Capacity
A ladder for rodents (AVS projects, São Carlos-SP) was used to carry out the RT protocol. The model was described by Frajacomo et al. (39), where the animals perform dynamic climbing movements with the help of their paws. A 30-cm2 box is attached to the top of the ladder for the animal to rest. The device used to place the load on each animal was adapted as described by Pereira et al. (34), using a conical plastic tube fixed with adhesive tape to the animal’s tail. Adaptation to the apparatus was performed for 4 days, as proposed by Cassilhas et al. (40). Initially, the animal was kept for 60 s inside the box with the device for placing empty loads attached to the tail. Subsequently, the climbing attempts took place gradually, the first with the animal positioned at 15 cm on the ladder, the second at 25 cm, and from the third, the animal was placed at the base of the ladder (50 cm) away from the box. After each attempt, a period of 60 s of rest was established. Trials at the base of the ladder continued until the animal performed three successful trials without the need for any type of stimulus. Forty-eight hours after the period of adaptation to the apparatus, the animals were submitted to the maximum voluntary load capacity test described by Pereira et al. (34). The load used in the RT protocol was determined from the maximum individual load each animal could carry in a 50 cm climbing series (41, 42).
RT Protocol
Forty-eight hours after maximum voluntary carrying capacity (MVCC) determination, the RT protocol began, performed shortly after the beginning of the TRF protocol, at the beginning of the animal’s light cycle (ZT0 to ZT1). The RT consisted of three weekly sessions using 70% of the MVCC training load for a period of 8 wk. The protocol was carried out on alternate days, that is, on one day the mice performed RT and on the other day, they remained without exercising. The increase in the number of climbs occurred progressively, with twenty series of one climb in the first week, twenty series of two climbs in the second week, and from the third to the eighth week, the mice performed twenty series of three climbs. At the end of each series, each animal had a 60-s rest. The RT protocol was adapted from Pereira et al. (34). No adverse results were observed in any of the animals during RT. The experimental design is summarized in Fig. 1, A and B.
Glucose Tolerance Test
In the seventh week, 24 h after the last RT session and with a 4-h fast, a small cut was made in the animal’s tail for blood collection. This time point was considered as time zero (t0) to measure the basal blood glucose. Subsequently, a 25% glucose solution (2 g/kg of body wt) was administered intraperitoneally (ip), with subsequent collection of blood samples at 30, 60, and 120 min for blood glucose measurement (Accu-Check Active/Roche, Switzerland). After the test, a compressive bandage (Johnson and Johnson, São Paulo, SP, Brazil) was used to stop the bleeding and the animals continued to be monitored for up to 3 h.
Insulin Tolerance Test
In the eighth week, 24 h after the last RT session and after a 4-h fast, a small cut was made in the animal’s tail for blood collection. This point was considered as time zero (t0) to measure the basal blood glucose. Subsequently, recombinant human insulin (Humulin R; 1.5 U/kg body wt) from Eli Lilly (Indianapolis, IN) was administered intraperitoneally (ip), with subsequent collection of blood samples at 5, 10, 15, 20, 25, and 30 min for blood glucose measurement (Accu-Check Active/Roche, Switzerland). To prevent bleeding, a compressive bandage (Johnson and Johnson, São Paulo, SP, Brazil) was used and the animals remained under monitoring for up to 3 h. The analysis was performed according to Bonora et al. (43).
Quantification of Blood Glucose
Twenty-four hours after the last RT session and after a 4-h fasting period, the blood was collected from the animals’ tail to measure fasting blood glucose using a glucometer (Accu-Chek Active/Roche, Switzerland). Blood samples of animals were centrifuged (1,100 g) for 10 min at 4°C, and the serum was stored at −80°C.
Comprehensive Laboratory Animal Monitoring System
The animals were placed in the Oxymax CLAMS (Comprehensive Laboratory Animal Monitoring System; Columbus Instruments), which allows the determination of energy expenditure through calorimetry. The animals underwent a 24-h period of adaptation to the equipment. Immediately after the adaptation period, animals underwent another 24 h of monitoring. The oxygen consumption (V̇o2), carbon dioxide consumption (V̇co2), respiratory exchange ratio (RER), and energy expenditure (EE) of animals were evaluated. The RT and TRF + RT groups were removed from the equipment during the physical training period, and returned immediately after the end of the protocol. Light and feeding conditions were similar to standard cages. To calculate the area under the curve, the values obtained during the CLAMS were used in each hour of the light and dark cycles (12/12 h). The first hour of the light cycle was added to the second hour of the same cycle, then this value obtained in the addition was divided by 2 and the quotient was multiplied by 1. This process continues until the penultimate hour of the cycle (11 h). In the last hour of the cycle (12 h), the values corresponding to all previous hours are added. The last hour’s value was used to plot the area under the curve. This process is carried out both in the light cycle and in the dark cycle (44).
Animal Euthanasia and Tissue Extraction
Twenty-four hours after the last RT session and with a 4-h fasting period, mice received intraperitoneal (ip) injections of ketamine chlorhydrate (90 mg/kg; Ketalar; Parke-Davis, Ann Arbor, MI) and xylazine (10 mg/kg; Rompun; Bayer, Leverkusen). Therefore, after the experimental period (8 wk), all mice underwent the procedure of anesthesia at the same circadian time, before being euthanized by decapitation (ZT1—07:00 AM). The samples of the adipose depots (i.e., perigonadal, inguinal, mesenteric, and retroperitoneal), gastrocnemius muscle, and liver were weighed on an analytical balance scale (GehakaBK300). The samples were frozen in liquid nitrogen and stored at −80°C until analysis. The liver tissue was fragmented for RT-qPCR, biochemistry, and histological techniques.
Bodyweight, Lee’s Index, and Relative Muscle Tissue Weight
The animals were weighed weekly on an analytical balance scale (L3102I, BEL) to monitor body weight evolution. The body weight gain of the animals was determined using the formula: Weight gain (g) = [Final weight (g) – Initial weight (g)]. The Lee Index was also carried out to calculate the weight gain of animals through the final weight of the animal at the end of the experiment, using the ratio between the cubic root of body weight and the naso-anal length of the animal [3√Weight(g)/CNA(cm)] as described by Novelli et al. (45). The relative tissue weight was determined by calculating the weight of muscle tissue (gastrocnemius muscle) (g)/body weight (g) × 100 as described by Bernardes et al. (46).
Coefficient of Weight Gain by Caloric Consumption
The coefficient of weight gain by calorie consumption (CWGCC, in kcal) was assessed through the relationship between weight gain and the amount of food consumed: CWGCC = (FW − IW)/kcal ingested, where FW is the final weight (g) of the animal at the end of the experimental period, IW is the body weight of the animal at the beginning of the experiment, and kcal is the total caloric value of the food ingested during the investigation (47). The feed was weighed daily on an analytical balance scale (L3102I, BEL) by monitoring the remaining weight of the food.
High-Resolution Respirometry
The hepatic consumption of oxygen was analyzed using the Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria), as previously described (48). The method used to control the calibration of the equipment was described by Nászai et al. (49). Approximately 50 mg of the dissected liver was homogenized using a pestle homogenizer in 0.5 mL of ice-cold MIR05 (110 mM sucrose, 60 mM K-lactobionate, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, pH 7.1 and 0. 1% BSA) at a concentration of 1 mg/10 μL and 2 mg (20 μL) of tissue were added to the oxygen chamber containing 2 mL of MIR05 buffer. Measurements were performed at 37°C. To assess complex I-dependent mitochondrial respiration, malate (0.1 mM) and glutamate (10 mM) were added to the chambers. To measure the respiratory stimulation of the fatty acid oxidation (FAO)-pathway, octanoylcarnitine (0.2 mM) and ADP (2.5 mM) were added. The combined respiration of complexes I and II was evaluated by the addition of succinate (10 mM). Subsequently, mitochondrial coupling was evaluated by inhibiting ATP synthase by adding oligomycin (2.5 μM). The maximum capacity of the electron transport system was measured by multiple titration steps of carbonyl cyanide p-trifluoromethoxy phenyl-hydrazone (FCCP) (0.5 μM).
Quantification of Fatty Acids
Nonesterified fatty acid in liver tissue was measured using the free fatty acid (FFA) kit (MAK044, Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s protocol. After the tissue was homogenized, 50 µL was added to the individual wells of the plate. Subsequently, an amount of 2 µL of acyl-CoA synthase was added to each well and incubated at 37°C for 30 min. Then, 50 µL of the master reaction mix (44 µL of fatty acid assay buffer, 2 μL of fatty acid probe, 2 μL of enzyme mix, and 2 μL of enhancer) were added and incubated again at 37°C for 30 min in the dark. The absorbances of individual wells were read at 570 nm using a microplate spectrophotometer (BioTek, Winooski, VT).
Oil Red Staining and Hematoxylin-Eosin
After liver extraction, part of the tissue was fixed in isopentane for cryopreservation at −80°C. The tissue was sectioned 7 µM longitudinally using the Leica Cryostat (CM1850). The slides were then stained with an oil red solution (Sigma-Aldrich, Brazil) for 25 min, counterstained with hematoxylin for 2 min, washed, and sealed with gelatin: glycerin solution. Images were obtained using Leica Application Suite software and the stained areas (red) were quantified using the ImageJ program (Bethesda, MD). For hematoxylin-eosin staining, slides were prepared and images were obtained with the Leica Application Suite software. The NAFLD Activity Score was assessed in a double-blind fashion to characterize diet-induced liver damage. Steatosis parameters were classified as score 0 (<5%), score 1 (5%–33%), score 2 (>33%–66%), and score 3 (>66%). For hepatocellular ballooning, score 0 (none), score 1 (few ballooning hepatocytes), and score 2 (many ballooning hepatocytes) were used. For lobular inflammation, score 0 (no foci), score 1 (2 foci per field), score 2 (2–4 foci per field), and score 3 (> 4 foci per field) were used. All parameters were used to calculate the NAFLD Activity Score (total score 0–8) (50, 51). The steatogenic profile was evaluated at ×400 magnification and inflammation (10 fields) was characterized as an inflammatory focus when a group of at least five inflammatory cells were not arranged in a row (52).
RT-qPCR
The extracted tissue (liver) was homogenized in 1,000 μL of Trizol (Life Technologies, Rockville, MD), and the RNA content was extracted according to the manufacturer’s instructions. A total of 2 μg of RNA was used for cDNA synthesis using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems), and cDNA samples were subjected to real-time quantitative polymerase chain reaction (RT-qPCR) using 50 ng cDNA and 0.4 μM of the following primers: Ppara, Cpt1a, Acsl1, Acox, Acadl, Fasn, ACC, Cd36, Fatp4, Srebp-1c, IL-1β, IL-6, Tnf-α, TRL4, NF-kB, and Gapdh for endogenous control synthesized by Exxtend (Paulínia, SP, Brazil). Real-time PCR was performed in triplicate with forward and reverse specific oligonucleotide primers in a final volume of 10 µL with PowerTrack SYBR Green PCR Master Mix (Thermo Fisher). The reaction was performed in 7500 Fast Real-Time PCR equipment (Applied Biosystems). The thermocycling conditions used were enzyme activation at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing, and extension at 60°C for 60 s. For data normalization, the Gapdh reference gene was previously tested and validated by the Best Keeper software. Messenger RNA (mRNA) levels were normalized by a housekeeping gene (Gapdh) using the ΔΔCt quantification method. The primer sequences are listed in Table 1.
Table 1.
Primer sequences used in the RT-qPCR technique
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Ppara | ACCACTACGGAGTTCACGCATG | GAATCTTGCAGCTCCGATCACAC |
| Cpt1a | AAAGATCAATCGGACCCTAGACA | CAGCGAGTAGCGCATAGTCA |
| Acsl1 | ACACTTCCTTGAAGCGATGG | GGCTCGACTGTATCTTGTGG |
| Acox | GGGAGTGCTACGGGTTACATG | CCGATATCCCCAACAGTGATG |
| Acadl | TCCATGGCAAAATACTGGGC | GCATCCACGTAAGCTTTTGC |
| Fasn | CCTCGGGTGAGGACGTTTAC | GTGCCGTGGGCGAGG |
| ACC | GTTCTGTTGGACAACGCCTTCAC | GGAGTCACAGAAGCAGCCCATT |
| Cd36 | TGGAGCTGTTATTGGTGCAG | TGGGTTTTGCACATCAAAGA |
| Fatp4 | GACTTCTCCAGCCGTTTCCACA | CAAAGGACAGGATGCGGCTATTG |
| Srebp-1c | GAGCCATGGATTGCACATTT | GGGAAGTCACTGTCTTGGTTG |
| IL-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| IL-6 | ACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| Tnf-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| TRL4 | GTTCTCTCATGGCCTCCACT | GGAACTACCTCTATGCAGGGAT |
| NF-kB | GATTCCGGGCAGTGACG | GATGAGGGGAAACAGATCGTCC |
| Gapdh | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Statistical Analysis
All results are presented as means ± standard deviation (SD). The Shapiro–Wilk test was used to examine normality and data that passed the normality test were analyzed using ANOVA, and two-way ANOVA when groups were compared at different times, followed by Tukey’s post hoc test. Data that did not pass the normality test were analyzed using the Kruskal–Wallis test complemented by the Dunn test. The GraphPad Prism 8.0.1 program was used to analyze and prepare graphs. All tests were two-sided with a level of significance set at 5%.
RESULTS
Resistance Training Increases Strength Performance
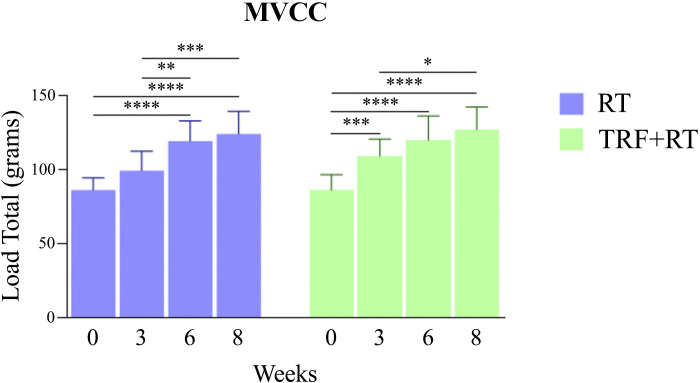
The gain in strength was evaluated throughout the experiment by measuring the maximum voluntary carrying capacity (MVCC) of the exercised groups (RT and TRF + RT) at baseline, and in weeks 3, 6, and 8. Results of the MVCC test show that performance improved over the course of the experiment in both groups. In the resistance-trained mice (RT, blue), a significant increase in performance was observed in the sixth week, and maintained until the end of the experiment. In mice submitted to combined TRF and RT (TRF + RT, green), a significant performance improvement was observed in the third week and also maintained until the end of the experiment (Fig. 2). At the end of the study, mice in both groups had similar MVCC values. Therefore, our RT protocol successfully increased animal strength in both groups.
Figure 2.
Maximum voluntary carrying capacity (MVCC) test. Test of maximum load carrying capacity of exercised animals performed at weeks 0, 3, 6, and 8 of the experimental protocol. Bars represent means ± SD, (n = 12 animals), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. RT, resistance exercise training; TRF, time-restricted feeding.
TRF Combined with RT Is More Effective in Preventing Weight Gain and Adiposity and in Regulating Glycemic Homeostasis than Each Intervention Alone
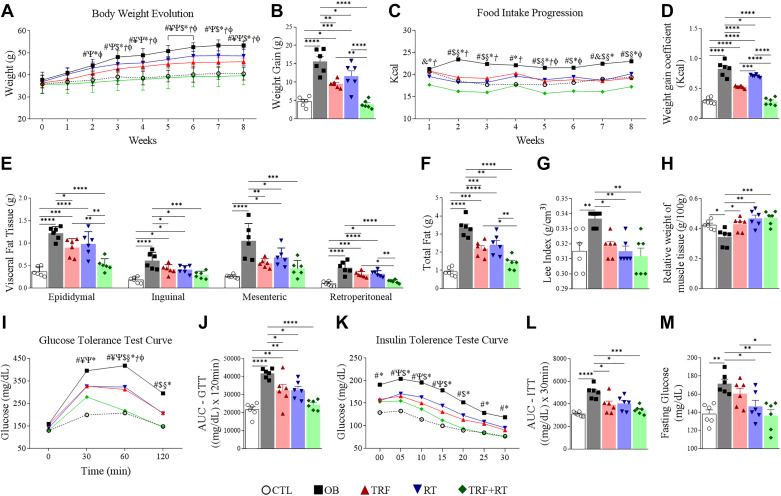
A significant difference in body weight between the OB and control group was present from the second week until the end of the protocol. Body weight was significantly lower in the TRF + RT group when compared with the OB group from the second week. The TRF group presented attenuated weight gain compared with the OB group from the third week onward (Fig. 3A). At the end of the 8-wk intervention, weight gain was significantly lower in the TRF + RT group than in the OB group and in the animals subjected to the individual TRF or RT interventions (Fig. 3B). Remarkably, weight gain was similar between animals fed a chow diet (CTL) and animals fed a high-fat diet submitted to TRF + RT.
Figure 3.
Time-restricted feeding (TRF) and resistance exercise training (RT) affect weight gain, food consumption, body adiposity, and glycemic homeostasis. Body weight progression (A); weight gain (B); food intake progression (C); weight gain coefficient (kcal) (D); visceral fat tissue (E); total fat (F); Lee index (G); relative fresh weight of gastrocnemius muscle (H); glucose tolerance test (GTT) curve (I); area under the curve (AUC) of the glucose tolerance test (GTT) (J); insulin tolerance test curve (K); area under the curve (AUC) of the insulin tolerance test (ITT) (L); fasting blood glucose (M). CTL, control group; OB, obese; RT, resistance exercise training; TRF, time-restricted feeding. The symbols represent statistics for #: control group (CTL) vs. obese (OB); ¥: CTL vs. TRF; Ψ: CTL vs. RT; &: CTL vs. TRF+RT; $: OB vs. TRF; §: OB vs. RT; *: OB vs. TRF+RT; Ꞓ: TRF vs. RT; Ϯ: TRF vs. TRF+RT; φ: RT vs. TRF+RT. Data are expressed as means ± SD, (n = 6 animals), P < 0.05. Bars represent means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
These differences in weight gain were associated with differences in food consumption, with the intervention groups (TRF, RT, and TRF + RT) having significantly lower food consumption than the OB group. In addition, the TRF + RT group had lower food consumption compared with the other isolated interventions (Fig. 3C). The coefficient of weight gain by caloric consumption (CWGCC) (Fig. 3D) was greater in the OB, TRF, and RT groups compared with the CTL group. However, the intervention groups (TRF, RT, and TRF + RT) showed a reduction compared with the OB group. In addition, the TRF + RT group showed a reduction compared with each of the isolated interventions. Notably, the TRF + RT group showed no difference from the CTL group.
Overall, the TRF, RT, and TRF + RT groups had less adiposity than the OB group. Furthermore, the TRF + RT had less fat than each of the TRF and RT alone (Fig. 3, E and F). Looking at individual fat depots (epididymal, inguinal, mesenteric, and retroperitoneal) suggested that the combined intervention (TRF + RT) more significantly reduced epididymal and retroperitoneal fat weight compared with each of the isolated interventions. The Lee Index (Fig. 3G) was higher in obese mice (OB) compared with control mice. On the contrary, the Lee index was lower in the groups submitted to isolated (TRF or RT) or combined interventions (TRF + RT) when compared with the obese group (OB). On the other hand, the OB group had a lower relative fresh weight of gastrocnemius muscle compared with the CTL group, and the groups submitted to isolated (TRF or RT) or combined interventions (TRF + RT) (Fig. 3H).
Finally, we assessed the effect of the different interventions on glucose homeostasis parameters. Glucose tolerance test (GTT) revealed a significant improvement in blood glucose regulation in the experimental groups submitted to the interventions (TRF, RT, and TRF + RT) compared with the OB group (Fig. 3, I and J). Similarly, insulin tolerance test (ITT; Fig. 3, K and L) showed better insulin response in the intervention groups than in the OB group. In fasting blood glucose (Fig. 3M), the intervention groups (TRF, RT, and TRF + RT) had lower levels than the OB group. In addition, the TRF + RT group had lower levels than the TRF group.
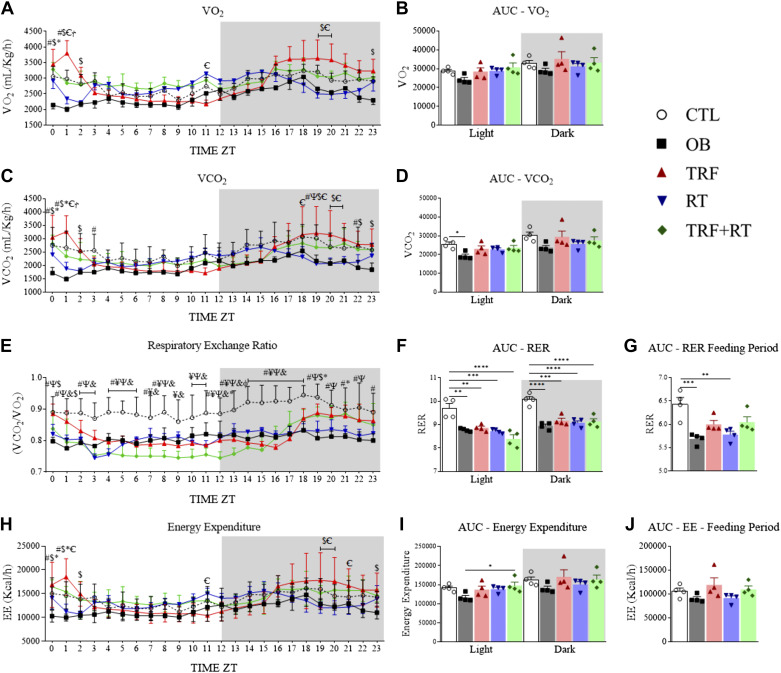
TRF Combined with RT Improved Respiratory Exchange Rate and Energy Expenditure
The mice were put in indirect calorimetry chambers after the seventh week of the experimental period to assess the effect of the different interventions on oxygen consumption, carbon dioxide production, respiratory exchange rate, and energy expenditure. The animals' oxygen consumption rate was not different between the groups in any biological cycles (light and dark) (Fig. 4, A and B). The carbon dioxide production rate was lower in the OB group than in the CTL group in the inactive cycle (Fig. 4, C and D). The analysis of the area under the curve of the respiratory exchange ratio (RER) showed that, in both biological cycles, the OB, TRF, RT, and TRF + RT groups had lower levels compared with the CTL group (Fig. 4, E and F) possibly due to the difference in diet. During the fed period, which occurred in the active phase of the mice, only the OB and RT groups presented lower levels of RER than the CTL group (Fig. 4G). The TRF + RT group showed higher energy expenditure than the OB group in the inactive period (Fig. 4, H–J).
Figure 4.
Time-restricted feeding (TRF) and resistance exercise training (RT) affect oxygen consumption, carbon dioxide production, respiratory exchange rate, and energy expenditure. Oxygen consumption (A); area under the oxygen consumption curve during the light and dark cycles (B); production of carbon dioxide (C); area under the carbon dioxide production curve during the light and dark cycles (D); respiratory exchange rate (RER, E); area under the respiratory exchange rate curve during the light and dark cycles (F); area under the respiratory exchange rate curve in the fed period (G); energy expenditure (EE, H); area under the energy expenditure curve during the light and dark cycles (I); area under the energy expenditure curve of the fed period (J). CTL, control group; OB, obese; RT, resistance exercise training; TRF, time-restricted feeding. The symbols represent statistics for #: control group (CTL) vs. obese (OB); ¥: CTL vs. TRF; Ψ: CTL vs. RT; &: CTL vs. TRF+RT; $: OB vs. TRF; §: OB vs. RT; *: OB vs. TRF+RT; Ꞓ: TRF vs. RT; Ϯ: TRF vs. TRF+RT; φ: RT vs. TRF+RT. Data are expressed as means ± SD, (n = 4 animals), P < 0.05. Bars represent means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. ZT, zeitgeber time.
TRF, RT, and Combined TRF + RT Prevented the Accumulation of Lipids and Improved Mitochondrial Respiration in the Liver
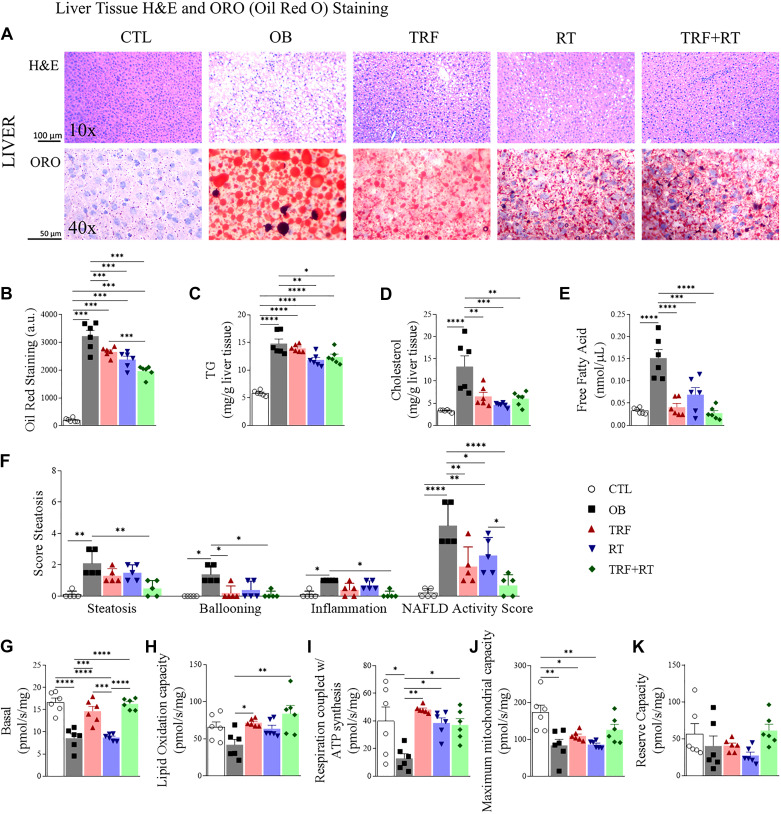
We performed hematoxylin-eosin (H&E) and Oil Red O staining of the liver to visualize lipid accumulation in the different intervention groups. Microscopic examination revealed the absence of lipids in the CTL group and the expected accumulation of lipids in the OB group. Qualitatively, lipid droplets appeared to be less and smaller in the TRF, RT, and TRF + RT groups (Fig. 5A). To confirm this observation, we quantified the oil red staining and, as expected, a greater amount of liver fat was observed in the groups fed a high-fat diet (OB, TRF, RT, and TRF + RT) in relation to the CTL group. On the other hand, the groups submitted to the isolated (TRF and RT) or combined interventions (TRF + RT) presented smaller amounts of hepatic fat in relation to the OB group. Furthermore, the TRF + RT group had lower levels of liver fat than the TRF group (Fig. 5B). To further confirm these observations, we quantified biochemically the amount of triglycerides (TG) in the liver. As expected, hepatic TG levels were lowest in the CTL group and highest in the OB group (Fig. 5C). The TRF, RT, and TRF + RT groups on a high-fat diet had higher TG levels than CTL, but the exercised groups (RT and TRF + RT) showed significantly lower TG levels compared with the OB group (Fig. 5C). We also quantified biochemically the liver cholesterol (Cho) level. Similar to TG levels, and as expected, hepatic Cho levels were lowest in the CTL group and highest in the OB group (Fig. 5D). Interestingly, the TRF, RT, and TRF + RT groups all had lower Cho levels than the OB group, that was almost normalized to the CTL group (Fig. 5D). Finally, we quantified free fatty acids. The results were similar to that of cholesterol, with the OB group having higher levels than the control group and the TRF, RT, and TRF + RT groups presenting significantly lower levels of free fatty acids than the OB group (Fig. 5E).
Figure 5.
Lipid profile and mitochondrial respiration in the liver. The histological plate of liver tissue stained with hematoxylin-eosin (H&E) and Oil Red (A); quantification of the area stained with Oil Red (n = 6 animals) (B); hepatic triglycerides (TG, n = 6 animals) (C); hepatic cholesterol (n = 6 animals) (D); quantification of free fatty acids (n = 6 animals) (E); the analysis of nonalcoholic fatty liver disease (NAFLD) Activity Score (n = 5 animals) (F); basal mitochondrial respiration (n = 6 animals) (G); lipid oxidation capacity (n = 6 animals) (H); respiration coupled with ATP synthesis (n = 6 animals) (I); maximum mitochondrial capacity (n = 6 animals) (J); reserve capacity (n = 6 animals) (K). Bars represent means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. CTL, control group; OB, obese; RT, resistance exercise training; TRF, time-restricted feeding.
The analysis of the steatosis score corroborates these histological and biochemical data. As expected, steatosis parameters were higher in the OB group compared with the CTL group. The combined TRF + RT group had a lower level of steatosis than the OB group. The OB group was greater than the CTL group in the hepatocellular ballooning parameter. On the other hand, the TRF and TRF + RT groups showed lower ballooning levels than the OB group, whereas the OB group was higher than the CTL group in the inflammation parameter. The combined TRF + RT group had a lower level of inflammation than the OB group. Thus, the NAFLD Activity Score has a higher level in the OB group than in the CTL group. The groups submitted to isolated (TRF and RT) or combined interventions (TRF + RT) had lower NAFLD Activity Score levels than the OB group. The combined TRF + RT group had even lower NAFLD Activity Score levels than the RT group (Fig. 5F).
We then evaluated the effect of the interventions (TRF, RT, and TRF + RT) on liver mitochondrial respiration. The basal mitochondrial respiration rate was lower in the OB and RT groups compared with the CTL group. The TRF and TRF + RT groups, on the other hand, showed significantly higher basal respiration rates than the OB and RT groups (Fig. 5G). A higher level of lipid oxidation capacity was found in the TRF and TRF + RT groups when compared with the OB group (Fig. 5H). Respiration coupled to ATP synthesis was higher in the CTL, TRF, RT, and TRF + RT groups than in the OB group (Fig. 5I). The CTL group showed a higher level of maximum mitochondrial capacity when compared with the OB, TRF, and RT groups (Fig. 5J). Finally, there was no difference between groups in the reserve capacity (Fig. 5K). Overall, TRF and RT showed improvement in mitochondrial respiration, however the combined intervention (TRF + RT) demonstrated no complementary effect compared with isolated interventions.
TRF, RT, and Combined TRF + RT Reduced Markers of Lipogenesis and Inflammation and Increased Lipid Oxidation in Liver Tissue
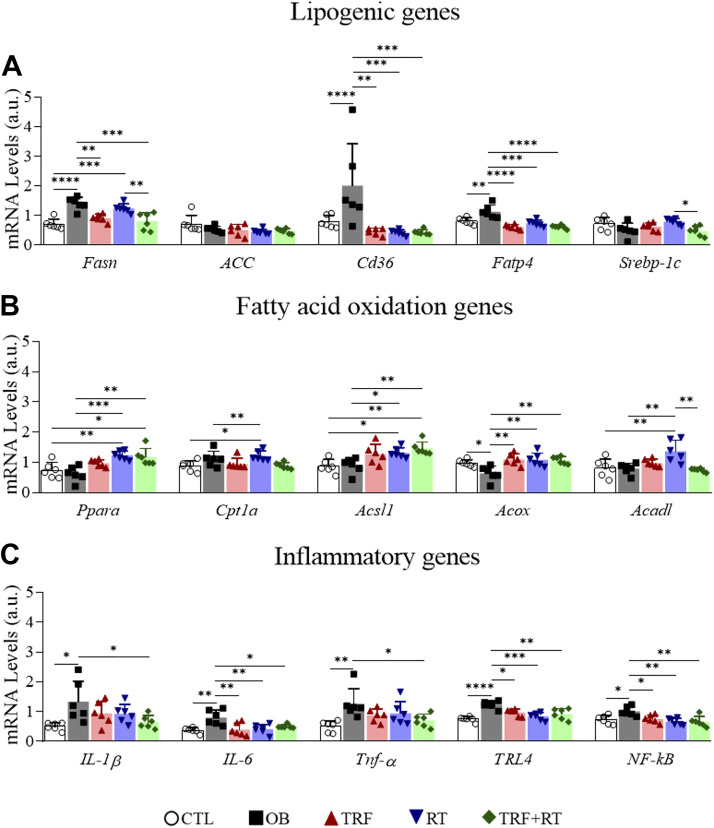
To gain an insight into the molecular alterations associated with the different interventions in the liver, we analyzed the mRNA expression of genes involved in lipogenesis (Fasn, ACC, Cd36, Fatp4, and Srebp-1c), fatty acid oxidation (Ppara, Cpt1a, Acsl1, Acox, and Acadl), and inflammation (IL-1β, IL-6, Tnf-α, TRL4, and NF-kB). The OB mice exposed to a high-fat diet ad libitum showed significantly increased expression of lipogenesis (Fasn, CD36, and Fatp4) and inflammatory genes (IL-1β, IL-6, Tnf-α, TRL4, and NF-kB). Overall, the groups submitted to the interventions (TRF, RT, and TRF + RT) presented a reduction in both the expression of lipogenesis and inflammation genes; whereas expression of fatty acid oxidation genes (Ppara, Acsl1, and Acox) was higher than in the OB group (Fig. 6, A–C).
Figure 6.
Gene Expression in the liver. Lipogenic genes (A); fatty acid oxidation genes (B); inflammatory genes (C). Bars represent means ± SD, (n = 6 animals), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. CTL, control group; OB, obese; RT, resistance exercise training; TRF, time-restricted feeding.
DISCUSSION
The present study investigated the impact of TRF and RT interventions alone or combined on body adiposity, glucose homeostasis, and hepatic metabolism in mice fed a high-fat diet. The combination of TRF with RT was more effective in attenuating weight gain and body adiposity, as well as improving glycemic homeostasis, than each intervention alone. We also showed that the TRF + RT group had lower lipogenesis and inflammation mRNA levels, higher fatty acid oxidation genes in the liver, and better performance in the MVCC test throughout the experiment. However, the reduction in lipid deposition in the liver of mice in the TRF + RT group was not greater than those found with each intervention alone (TRF or RT). Altogether, these findings suggest that the combination of TRF and RT positively impacted metabolic health and reduced the harmful effects of high-fat-diet feeding, and that the interventions can have complementary benefits on some metabolic parameters but not on others.
Both TRF (53, 54) and RT (55, 56) have previously been shown to independently prevent weight gain in rodents fed a high-fat diet. Likewise, clinical studies have shown that both TRF (57, 58) and RT (59, 60) can cause weight loss. Here, we show the complementary benefits of combining TRF with RT to further prevent weight gain, which was associated with a significant reduction in fat mass accumulation in selective depots (epididymal, retroperitoneal, and total fat mass) compared with each intervention alone. Differences in weight gain under TRF have been associated with increased energy expenditure in the absence of changes in energy intake in some studies (53, 61), whereas other studies found a reduction in energy intake (54, 62). For RT, studies showed that increased energy expenditure contributed to weight loss (63, 64). In agreement with the literature, our study showed weight gain prevention in both the TRF and RT groups independently.
Analysis of the gastrocnemius muscle indicated that obese mice had lower weight of the muscle compared with control mice. The study by Bhatt et al. (65) demonstrated an association between obesity, muscle loss, and dysfunction, as well as the development of muscle atrophy. Likewise, rodents fed a high-fat diet showed structural, inflammatory, and protein synthesis changes, typical features of muscle wasting, such as weakness, reduced muscle mass, and decreased muscle fiber diameter (66–69). These data corroborate a study by Tang et al. (70) that showed a reduction in lean mass in rodents fed an HFD compared with control animals. On the other hand, in our study, mice that underwent TRF and TRF + RT presented preserved muscle mass weight. Studies that used TRF as a strategy to mitigate the negative impacts of a high-fat diet showed beneficial effects on metabolic function and muscle physiology in animals (53, 71–74) and also in humans (75). Another study, using mice under a western diet verified that TRF benefits included an extension of muscle performance, motor coordination, and glucose regulation (76). Furthermore, the rhythm of protein synthesis through mammalian target of rapamycin complex 1 (mTORC1) and its associated amino acids was increased under a time-restricted regime in mice (61). In addition, studies relating the application of TRF with aging have found that this strategy can lead to anabolic sensitivity, improved insulin sensitivity, and increased uptake of amino acids, allowing improved muscle protein synthesis (77–80).
However, this is an issue that deserves special attention in future studies. Recently, a study by Chow et al. (81) reported results obtained through dual X-ray absorptiometry (DXA) in obese adults, and found greater loss of total body weight, visceral fat, and lean mass in the TRF group compared with the non-TRF group. The loss of lean mass in the TRF group was in the legs, which was significant compared with preintervention and non-TRF. TRF did not result in considerable trunk or arm lean mass loss. The TRF intervention did not change insulin sensitivity relative to the preintervention or non-TRF group. It should be noted that there was a low number of participants (17 women/3 men), and that, therefore, the interpretation of these data should be performed with caution.
The TRF + RT group had the lowest food consumption. In addition, our indirect calorimetry data demonstrated that the TRF and TRF + RT groups presented lower respiratory exchange during the period of food restriction, but higher respiratory exchange during the period of exposure to food, suggesting greater metabolization of lipids in periods of food deprivation and greater intake of carbohydrates in the feeding periods for energy production. Similar results in the literature indicate greater glycolysis and fat oxidation in response to TRF (14, 53, 74).
The preventive effect on weight gain seen in the TRF and TRF + RT groups may also be related to lower cumulative calorie intake, since the TRF + RT group had the lowest food consumption throughout the experiment. Importantly, consolidating food access to one period of the day may constitute an efficient strategy to reduce appetite and/or food intake in both humans and rodents (53, 82–86). Whether this is the case under TRF has not been thoroughly investigated. A previous study showed that intermittently fasted rats (fed with a standard diet) presented higher neuropeptide Y (NPY) and agouti-related peptide (AgRP) levels in the hypothalamus when fed and fasted, leading to overeating when food is available (83). The authors also demonstrated that the rats had higher levels of lipid oxidation on fasting days and higher metabolic rates during feeding days, with consequent lower efficiency of energy conversion, leading to a reduction in body mass through fasting (83). However, in rodents fed the high-fat diet, intermittent fasting may be an adjuvant treatment to improve the reestablishment of hypothalamic responses in obesity conditions (87). The effect of TRF + RT on appetite was not explored in our study and needs further investigation in future research. In all, combined changes in intake and energy expenditure likely explain the complementary effect of TRF and RT in preventing weight gain.
We confirmed previous results of the benefits of TRF and RT on insulin sensitivity and glycemic homeostasis. We show that high-fat diet feeding increases circulating glucose levels, observed in the OB group, as previously described (88, 89). In addition, high-fat diet consumption impairs insulin signaling in the liver and induces metabolic alterations, exacerbating glucose production and lipogenesis (14, 37), and triggering hepatic fat accumulation (8). Previous results obtained by our research group (14) corroborate the current findings. On the other hand, TRF or RT alone, or the combination of TRF + RT was able to increase insulin sensitivity and improve glycemic homeostasis in mice. There is abundant evidence that TRF contributes to increased insulin sensitivity and reduced blood glucose in rodents fed a high-fat diet (10, 12). Resistance physical exercise also affects carbohydrate metabolism, with increased insulin responsiveness and decreased glycemia in obese rodents (28, 56). Importantly, in our combined intervention, TRF combined with RT significantly lowered fasting blood glucose when compared with the isolated effects of TRF. Previously, we demonstrated that the combination of TRF with aerobic exercise training provided more potent effects on glycemic homeostasis (14). Overall, our results support the use of this nonpharmacological strategy to improve glycemic homeostasis, insulin sensitivity, and hepatic metabolism in mice fed a high-fat diet.
Our study showed that a high-fat diet caused harmful histological alterations in the liver of mice, as indicated by the NAFLD Activity Score. However, the use of the interventions in our study, (TRF, RT, and TRF + RT) prevented the increase in NAFLD Activity Score in the rodents. This highlights the potential of these approaches to mitigate the effects of hepatic steatosis caused by a high-fat diet. We also show that TRF and RT prevented liver steatosis by biochemical (TG, cholesterol, and free fatty acid quantification) and histological (H&E and Oil Red) analyses of the liver. Mice submitted to TRF and RT alone or to TRF + RT showed less fat accumulation and an improved lipid profile in the hepatic tissue, demonstrating a protective phenotype compared with the OB group. These results are consistent with previous studies showing TRF-associated improvements in hepatic lipids (11, 14, 53) and with the literature showing that resistance exercise also attenuates the accumulation of fat in the liver (34). In a systematic review study, Hashida et al. (19) emphasize the relevance of resistance exercise in improving NAFLD, demonstrating that this type of activity may be more viable than aerobic exercise for patients with NAFLD with low cardiorespiratory fitness or for those who cannot tolerate or participate in aerobic exercise. Resistance exercise was also previously associated with body fat reduction, inflammation suppression, and insulin sensitivity enhancement in obese mice. Even when there is no significant change in body weight, resistance training can act positively by reducing the accumulation of fat in the liver of rodents fed a high-fat diet (90, 91). However, the combination of TRF with RT did not further reduce liver fat accumulation compared with TRF and RT alone. Perhaps with a longer period of intervention, it would be possible to find even more significant changes with the combined interventions (TRF + RT).
Mitochondrial dysfunction is one of the well-described pathophysiological adaptations to a high-fat diet (92). Overall, TRF effects on mitochondrial respiration in the liver are not yet clearly determined. The current study bridges this gap in the knowledge and demonstrates that the isolated interventions (TRF and RT) and their combination (TRF + RT) promoted improvements in mitochondrial respiratory function (increase in lipid oxidation capacity and respiration coupled to ATP synthesis) in the liver of mice. In addition, the TRF groups (TRF and TRF + RT) showed preserved basal mitochondrial respiration compared with the OB group. The impact of a high-fat diet on mitochondrial respiratory capacity is an intriguing question. With high fat feeding there is usually a compensatory effect of the mitochondria, where respiration capacity is increased. After very long periods of time, this compensation in the mitochondria fails (93). However, the results observed in the literature are sometimes conflicting and seem to depend on the animal model, exposure time, and type of diet used. Zhao et al. (94) investigated the association between mitochondrial dysfunction and the progression of fatty liver disease associated with metabolic dysfunction by analyzing several points in time (weeks 4, 8, 12, and 18) in 5-wk-old mice fed a standard or HFD diet. The authors found that already in the first 4 wk, hepatic mitochondrial oxygen consumption decreased in HFD fed mice compared to mice fed a standard diet. The study by Koliaki et al. (95), which evaluated the mitochondrial respiratory capacity using liver biopsies from lean healthy patients, obese patients with and without steatosis, and obese patients with inflammatory steatosis (NASH), observed that mitochondrial respiratory capacity was increased in obese individuals with and without liver fat compared with lean patients, although there was no change in mitochondrial mass.
On the other hand, the interventions used in our study (TRF, RT, and TRF + RT) minimized the damage to hepatic mitochondrial respiration caused by feeding a high-fat diet. Corroborating our findings, Chausse et al. demonstrated that intermittent fasting increases respiratory capacity in rat livers (96). Furthermore, it is known that exercise training also activates systemic pathways that indirectly modulate liver mitochondria bioenergetics, function, and structure (97, 98). Several studies confirm the positive relevance of a physically active lifestyle in the adaptation of liver mitochondria against the harmful consequences of NAFLD (99–103).
At the molecular level, the TRF + RT group showed a more significant reduction in the expression of lipogenesis (Fasn, Cd36, and Fatp4) and inflammation (IL-1β, IL-6, Tnf-α, TLR4, and NF-kB) genes while increasing the expression of fatty acid oxidation genes (Ppara, Acsl1, and Acox) than each of the interventions alone. The reduction in fatty acid synthesis and hepatic fat accumulation induced by TRF was previously described (10, 83). The isolated RT protocol was also able to attenuate inflammatory cytokines (Tnf-α e IL-1β) and lipogeneses genes (Fasn and Scd1) while increasing fatty acid oxidation genes (Ppara and Cpt1a), improving glycemic homeostasis, and enhancing insulin sensitivity in the liver of obese mice (34). A previous study demonstrated that resistance exercise could improve NAFLD, with less energy consumption than aerobic exercise (19). In addition, resistance training decreased the expression of lipogenic genes, such as sterol regulatory element binding protein-1c (SREBP-1c), acetyl-CoA carboxylase (ACC), and stearoyl-CoA desaturase-1 (SCD1), in the liver of ovariectomized rats (104). TRF alone was also effective in attenuating hepatic steatosis in obesity models (10).
In a previous study from our group (14), we evaluated the effect of TRF combined with aerobic exercise on weight gain and metabolic disease prevention under high-fat diet feeding. In this study, mice were fed for 8 h during the dark/active phase and aerobic exercise was performed at the end of the feeding period, in the light/inactive phase between ZT0 and ZT1 (60 min at a 60% exhaust speed) every day of the week (7 days/wk) for 10 wk. The study showed that there was a reduction in the damage caused by the high-fat diet, a decrease in body adiposity, and an improvement in hepatic metabolism and glucose intolerance. A study with obese humans submitted to a combined protocol of time-restricted feeding (on alternate days) with aerobic physical exercise (5 days a week) demonstrated significantly reduced hepatic steatosis and body weight. However, the effects were not more significant than those found with the adoption of TRF alone (36). In this manuscript, TRF was combined with RT performed on alternate days. This strategy could be advantageous to adherence and execution, considering future translational studies. Furthermore, future studies may test the effects of TRF combined with RT for longer periods and on different day cycles. The application of the TRF protocol combined with RT also needs to be tested in female mice, to see if there are differences between the sexes.
This study has some limitations. It should be mentioned that this is an experimental and descriptive study, without any intervention capable of determining mechanisms directly related to the combined effects of TRF and RT. The use of young animals (6 wk old) may influence the results due to the rapid growth of these animals. In future studies, the use of mature adult animals (8 wk old or older) from the beginning of the experiment should be considered. Another limitation is the small number of mice in the groups in the analysis of our study (4–6 animals/group), which could impact the statistical significance of some relevant parameters, such as respirometry, calorimetry, and glycemic and molecular parameters. It is relevant to consider that the results of the present study were obtained with mice maintained at a normal “cold” temperature (21 ± 2°C) and that future studies should consider the analysis at a thermoneutral temperature (29–31°C), since this is a variable that has significant impacts on mitochondrial function, energy expenditure, and food intake (105). Moreover, it is crucial to mention that physical exercise has multi-organic effects, with favorable actions on metabolism. Taking this into account, the more pronounced improvement in glycemic homeostasis found with the combined intervention (TRF + RT) may be related to the effects of exercise on improved glucose uptake capacity in other tissues, such as skeletal muscle and white adipose tissue (106–108). Therefore, the beneficial adaptations of this investigation may be linked to the effects of resistance exercise training on other peripheral tissues, which should be considered in future studies.
In conclusion, the findings of the present study highlight the potential benefits for metabolic health when TRF and RT strategies are used together. Furthermore, it is possible to consider that one of the interventions could compensate for the lesser effectiveness of the other or that both interventions (TRF + RT) have complementary actions in some synergistic signaling pathways. Therefore, TRF combined with resistance exercise training prevents obesity and improves lipid metabolism in the liver of young mice fed a high-fat diet.
DATA AVAILABILITY
The data supporting this study's findings are available from the corresponding author upon reasonable request.
GRANTS
This work was supported by the National Council for Scientific and Technological Development (CNPq) Case Numbers 311939/2020-1; 308999/2022-3, Coordination for the Improvement of Higher Education Personnel (CAPES; finance code 001), and São Paulo Research Foundation (FAPESP) Case Numbers 2022/08930-6; 2020/13443-1; 2019/11820-5. A.C. is supported by NIA Grant AG065993.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.d.L. and J.R.P. conceived and designed research; R.D.d.L., R.F.L.V., V.R.M., A.P.A.M., G.C.A., R.R.B., and S.C.B.R.M. performed experiments; R.D.d.L., J.R.P. and M.F. analyzed data; R.D.d.L., A.C., R.C.G., A.S.R.d.S., D.E.C., L.P.d.M., R.A.M., E.R.R., and J.R.P. interpreted results of experiments; R.D.d.L. prepared figures; R.D.d.L. and J.R.P. drafted manuscript; A.S.R.d.S., A.C., M.F., D.E.C., L.P.d.M., R.A.M., E.R.R., and J.R.P. edited and revised manuscript; R.D.d.L., R.F.L.V., V.R.M., A.C., A.P.A.M., G.C.A., M.F., R.R.B., S.C.B.R.N., R.C.G., A.S.R.d.S., D.E.C., L.P.d.M., R.A.M., E.R.R., and J.R.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract created with BioRender and published with permission.
REFERENCES
- 1. Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi'i A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med 136: 104754, 2021. doi: 10.1016/j.compbiomed.2021.104754. [DOI] [PubMed] [Google Scholar]
- 2. Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep 4: 363–370, 2015. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 3. Shaharir SS, Gafor AHA, Said MSM, Kong NCT. Steroid-induced diabetes mellitus in systemic lupus erythematosus patients: analysis from a Malaysian multi-ethnic lupus cohort. Int J Rheum Dis 18: 541–547, 2015. doi: 10.1111/1756-185X.12474. [DOI] [PubMed] [Google Scholar]
- 4. Kushner RF. Weight loss strategies for treatment of obesity: lifestyle management and pharmacotherapy. Prog Cardiovasc Dis 61: 246–252, 2018. doi: 10.1016/j.pcad.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, Kaptoge S, Di Angelantonio E, Stampfer M, Willett WC, Hu FB. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 138: 345–355, 2018. [Erratum in Circulation 138: e75, 2018]. doi: 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013: 139239, 2013. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15: 11–20, 2018. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 8. Thyfault JP, Scott Rector R. Exercise combats hepatic steatosis: potential mechanisms and clinical implications. Diabetes 69: 517–524, 2020. doi: 10.2337/dbi18-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, Wang R. Exercise as a prescription for patients with various diseases. J Sport Health Sci 8: 422–441, 2019. doi: 10.1016/j.jshs.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20: 991–1005, 2014. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr 39: 291–315, 2019. doi: 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Regmi P, Chaudhary R, Page AJ, Hutchison AT, Vincent AD, Liu B, Heilbronn L. Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice. J Endocrinol 248: 75–86, 2021. doi: 10.1530/JOE-20-0404. [DOI] [PubMed] [Google Scholar]
- 13. Aouichat S, Chayah M, Bouguerra-Aouichat S, Agil A. Time-restricted feeding improves body weight gain, lipid profiles, and atherogenic indices in cafeteria-diet-fed rats: role of browning of inguinal white adipose tissue. Nutrients 12: 2185, 2020. doi: 10.3390/nu12082185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vieira RFL, Muñoz VR, Junqueira RL, de Oliveira F, Gaspar RC, Nakandakari SCBR, Costa S de O, Torsoni MA, da Silva ASR, Cintra DE, de Moura LP, Ropelle ER, Zaghloul I, Mekary RA, Pauli JR. Time-restricted feeding combined with aerobic exercise training can prevent weight gain and improve metabolic disorders in mice fed a high-fat diet. J Physiol 600: 797–813, 2022. doi: 10.1113/JP280820. [DOI] [PubMed] [Google Scholar]
- 15. Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 16. Yang Y, Li X, Liu Z, Ruan X, Wang H, Zhang Q, Cao L, Song L, Chen Y, Sun Y. Moderate treadmill exercise alleviates NAFLD by regulating the biogenesis and autophagy of lipid droplet. Nutrients 14: 4910, 2022. doi: 10.3390/nu14224910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muñoz VR, Gaspar RC, Mancini MCS, de Lima RD, Vieira RFL, Crisol BM, Antunes GC, Trombeta JCS, Bonfante ILP, Simabuco FM, da Silva ASR, Cavaglieri CR, Ropelle ER, Cintra DE, Pauli JR. Short-term physical exercise controls age-related hyperinsulinemia and improves hepatic metabolism in aged rodents. J Endocrinol Invest 46: 815–827, 2023. doi: 10.1007/s40618-022-01947-8. [DOI] [PubMed] [Google Scholar]
- 18. Bae JY. Resistance exercise regulates hepatic lipolytic factors as effective as aerobic exercise in obese mice. Int J Environ Res Public Health 17: 8307–8309, 2020. doi: 10.3390/ijerph17228307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, Ueno T, Koga H, George J, Shiba N, Torimura T. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 66: 142–152, 2017. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 20. Cassidy S, Thoma C, Hallsworth K, Parikh J, Hollingsworth KG, Taylor R, Jakovljevic DG, Trenell MI. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 59: 56–66, 2016. doi: 10.1007/s00125-015-3741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJA, Richardson P, Kemp GJ, Barrett M, Jackson NC, Thomas EL, Bell JD, Umpleby AM. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 130: 93–104, 2016. doi: 10.1042/CS20150447. [DOI] [PubMed] [Google Scholar]
- 22. Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, Harrison SA. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol 6: 249–259, 2013. doi: 10.1177/1756283X13484078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond) 129: 1097–1105, 2015. doi: 10.1042/CS20150308. [DOI] [PubMed] [Google Scholar]
- 24. Finucane FM, Sharp SJ, Purslow LR, Horton K, Horton J, Savage DB, Brage S, Besson H, De Lucia Rolfe E, Sleigh A, Martin HJ, Aihie Sayer A, Cooper C, Ekelund U, Griffin SJ, Wareham NJ. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: a randomised controlled trial. Diabetologia 53: 624–631, 2010. doi: 10.1007/s00125-009-1641-z. [DOI] [PubMed] [Google Scholar]
- 25. Zhang H-J, He J, Pan L-L, Ma Z-M, Han C-K, Chen C-S, Chen Z, Han H-W, Chen S, Sun Q, Zhang J-F, Li Z-B, Yang S-Y, Li X-J, Li X-Y. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease. JAMA Intern Med 176: 1074–1082, 2016. doi: 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 26. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, Trenell MI. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60: 1278–1283, 2011. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, Kis O, Fliss-Isakov N, Izkhakov E, Halpern Z, Santo E, Oren R, Shibolet O. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol 20: 4382–4392, 2014. doi: 10.3748/wjg.v20.i15.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Botezelli JD, Coope A, Ghezzi AC, Cambri LT, Moura LP, Scariot PPM, Gaspar RS, Mekary RA, Ropelle ER, Pauli JR. Strength training prevents hyperinsulinemia, insulin resistance, and inflammation independent of weight loss in fructose-fed animals. Sci Rep 6: 31106, 2016. doi: 10.1038/srep31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology 58: 1287–1295, 2013. doi: 10.1002/hep.26393. [DOI] [PubMed] [Google Scholar]
- 30. Mekary RA, Grøntved A, Despres J, De Moura LP, Asgarzadeh M, Willett WC, Rimm EB, Giovannucci E, Hu FB. Weight training, aerobic physical activities, and long-term waist circumference change in men. Obesity (Silver Spring) 23: 461–467, 2015. doi: 10.1002/oby.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25, Suppl 3: 1–72, 2015. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 32. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muñoz VR, Gaspar RC, Kuga GK, Nakandakari SCBR, Baptista IL, Mekary RA, da Silva ASR, de Moura LP, Ropelle ER, Cintra DE, Pauli JR. Exercise decreases CLK2 in the liver of obese mice and prevents hepatic fat accumulation. J Cell Biochem 119: 5885–5892, 2018. doi: 10.1002/jcb.26780. [DOI] [PubMed] [Google Scholar]
- 34. Pereira RM, Rodrigues K. C D C, Anaruma CP, Sant'Ana MR, de Campos TDP, Gaspar RS, Canciglieri RDS, de Melo DG, Mekary RA, da Silva ASR, Cintra DE, Ropelle ER, Pauli JR, de Moura LP. Short-term strength training reduces gluconeogenesis and NAFLD in obese mice. J Endocrinol 241: 59–70, 2019. doi: 10.1530/JOE-18-0567. [DOI] [PubMed] [Google Scholar]
- 35. Nikroo H, Hosseini SRA, Fathi M, Sardar MA, Khazaei M. The effect of aerobic, resistance, and combined training on PPAR-α, SIRT1 gene expression, and insulin resistance in high-fat diet-induced NAFLD male rats. Physiol Behav 227: 113149, 2020. doi: 10.1016/j.physbeh.2020.113149. [DOI] [PubMed] [Google Scholar]
- 36. Ezpeleta M, Gabel K, Cienfuegos S, Kalam F, Lin S, Pavlou V, Song Z, Haus JM, Koppe S, Alexandria SJ, Tussing-Humphreys L, Varady KA. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab 35: 56–70.e3, 2023. doi: 10.1016/j.cmet.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, de Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One 7: e30571, 2012. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sundaram S, Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res 36: 603–611, 2016. doi: 10.1016/j.nutres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 39. Frajacomo FT, Kannen V, Deminice R, Geraldino TH, Pereira-Da-Silva G, Uyemura SA, Jordão AA Jr, Garcia SB. Aerobic training activates interleukin 10 for colon anticarcinogenic effects. Med Sci Sports Exerc 47: 1806–1813, 2015. doi: 10.1249/MSS.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 40. Cassilhas RC, Lee KS, Fernandes J, Oliveira MGM, Tufik S, Meeusen R, De Mello MT. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 202: 309–317, 2012. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 41. Hornberger TA, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol 29: 16–31, 2004. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- 42. Speretta GF, Silva AA, Vendramini RC, Zanesco A, Delbin MA, Menani JV, Bassi M, Colombari E, Colombari DSA. Resistance training prevents the cardiovascular changes caused by high-fat diet. Life Sci 146: 154–162, 2016. doi: 10.1016/j.lfs.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 43. Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M. Estimates of in vivo insulin action in man: Comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab 68: 374–378, 1989. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- 44. Mina AI, LeClair RA, LeClair KB, Cohen DE, Lantier L, Banks AS. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab 28: 656–666.e1, 2018. doi: 10.1016/j.cmet.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Novelli ELB, Diniz YS, Galhardi CM, Ebaid GMX, Rodrigues HG, Mani F, Fernandes AAH, Cicogna AC, Novelli Filho JLVB. Anthropometrical parameters and markers of obesity in rats. Lab Anim 41: 111–119, 2007. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 46. Bernardes D, Oliveira-Lima OC, da Silva TV, Juliano MA, dos Santos DM, Carvalho-Tavares J. Metabolic alterations in experimental autoimmune encephalomyelitis in mice: effects of prior physical exercise. Neurophysiology 48: 117–121, 2016. doi: 10.1007/s11062-016-9577-7. [DOI] [Google Scholar]
- 47. Nery C, da S, Pinheiro IL, de Muniz GS, de Vasconcelos DAA, de França SP, Nascimento ED. Murinometric evaluations and feed efficiency in rats from reduced litter during lactation and submitted or not to swimming exercise. Rev Bras Med Esporte 17: 49–55, 2011. doi: 10.1590/S1517-86922011000100010. [DOI] [Google Scholar]
- 48. Braga RR, Crisol BM, Brícola RS, Sant'ana MR, Nakandakari SCBR, Costa SO, Prada PO, da Silva ASR, Moura LP, Pauli JR, Cintra DE, Ropelle ER. Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPRmt in the hypothalamus of mice. Sci Rep 11: 3813, 2021. doi: 10.1038/s41598-021-82352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nászai A, Terhes E, Kaszaki J, Boros M, Juhász L. Ca(2+)N it be measured? Detection of extramitochondrial calcium movement with high-resolution fluorespirometry. Sci Rep 9: 19229, 2019. doi: 10.1038/s41598-019-55618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu Y-C, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 51. Guerra N, Leyens K, Müller L, Brauer D, Janowitz D, Schlick S, Pilz K, Grabe HJ, Vollmar B, Kuhla A. The effect of different weight loss strategies to treat non-alcoholic fatty liver disease focusing on fibroblast growth factor 21. Front Nutr 9: 935805–935814, 2022. doi: 10.3389/fnut.2022.935805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liebig M, Hassanzada A, Kämmerling M, Genz B, Vollmar B, Abshagen K. Microcirculatory disturbances and cellular changes during progression of hepatic steatosis to liver tumors. Exp Biol Med (Maywood) 243: 1–12, 2018. doi: 10.1177/1535370217738730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15: 848–860, 2012. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 26: 3493–3502, 2012. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 55. Guedes JM, da Pieri BS, Luciano TF, de Marques SO, Guglielmo LGA, de Souza CT. Muscular resistance, hypertrophy and strength training equally reduce adiposity, inflammation and insulin resistance in mice with diet-induced obesity. Einstein (Sao Paulo) 18: eAO4784, 2020. doi: 10.31744/einstein_journal/2020AO4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Minuzzi LG, Kuga GK, Breda L, Gaspar RC, Muñoz VR, Pereira RM, Botezelli JD, da Silva ASR, Cintra DE, de Moura LP, Ropelle ER, Pauli JR. Short-term resistance training increases APPL1 content in the liver and the insulin sensitivity of mice fed a long-term high-fat diet. Exp Clin Endocrinol Diabetes 128: 30–37, 2020. doi: 10.1055/a-0885-9872. [DOI] [PubMed] [Google Scholar]
- 57. Domaszewski P, Konieczny M, Dybek T, Łukaniszyn-Domaszewska K, Anton S, Sadowska-Krępa E, Skorupska E. Comparison of the effects of six-week time-restricted eating on weight loss, body composition, and visceral fat in overweight older men and women. Exp Gerontol 174: 112116, 2023. doi: 10.1016/j.exger.2023.112116. [DOI] [PubMed] [Google Scholar]
- 58. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 31: 92–104.e5, 2020. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dunstan DW, Daly RM, Owen N, Jolley D, de Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 25: 1729–1736, 2002. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 60. Hunter GR, Byrne NM, Sirikul B, Fernández JR, Zuckerman PA, Darnell BE, Gower BA. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity (Silver Spring) 16: 1045–1051, 2008. doi: 10.1038/oby.2008.38. [DOI] [PubMed] [Google Scholar]
- 61. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29: 303–319.e4, 2019. doi: 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gallop MR, Tobin SY, Chaix A. Finding balance: understanding the energetics of time-restricted feeding in mice. Obesity (Silver Spring) 31, Suppl 1: 22–39, 2023. doi: 10.1002/oby.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mazzetti S, Douglass M, Yocum A, Harber M. Effect of explosive versus slow contractions and exercise intensity on energy expenditure. Med Sci Sports Exerc 39: 1291–1301, 2007. doi: 10.1249/mss.0b013e318058a603. [DOI] [PubMed] [Google Scholar]
- 64. Petridou A, Siopi A, Mougios V. Exercise in the management of obesity. Metabolism 92: 163–169, 2019. doi: 10.1016/j.metabol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 65. Bhatt BA, Dube JJ, Dedousis N, Reider JA, O'Doherty RM. Diet-induced obesity and acute hyperlipidemia reduce IκBα levels in rat skeletal muscle in a fiber-type dependent manner. Am J Physiol Regul Integr Comp Physiol 290: R233–R240, 2006. doi: 10.1152/ajpregu.00097.2005. [DOI] [PubMed] [Google Scholar]
- 66. Sishi B, Loos B, Ellis B, Smith W, Du Toit EF, Engelbrecht A-M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol 96: 179–193, 2011. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 67. Dhillon RJS, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med 33: 17–26, 2017. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316: 129–139, 2010. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 69. Gatineau E, Savary-Auzeloux I, Migné C, Polakof S, Dardevet D, Mosoni L. Chronic intake of sucrose accelerates sarcopenia in older male rats through alterations in insulin sensitivity and muscle protein synthesis. J Nutr 145: 923–930, 2015. doi: 10.3945/jn.114.205583. [DOI] [PubMed] [Google Scholar]
- 70. Tang L, Gao X, Yang X, Liu C, Wang X, Han Y, Zhao X, Chi A, Sun L. Ladder-climbing training prevents bone loss and microarchitecture deterioration in diet-induced obese rats. Calcif Tissue Int 98: 85–93, 2016. doi: 10.1007/s00223-015-0063-9. [DOI] [PubMed] [Google Scholar]
- 71. Lettieri-Barbato D, Cannata SM, Casagrande V, Ciriolo MR, Aquilano K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS One 13: e0195912, 2018. doi: 10.1371/journal.pone.0195912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Olsen MK, Choi MH, Kulseng B, Zhao C-M, Chen D. Time-restricted feeding on weekdays restricts weight gain: a study using rat models of high-fat diet-induced obesity. Physiol Behav 173: 298–304, 2017. doi: 10.1016/j.physbeh.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 73. Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, Le HD, Manor U, Panda S, Melkani GC. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat Commun 10: 2700, 2019. [Erratum in Nat Commun 11: 2521, 2020]. doi: 10.1038/s41467-019-10563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Woodie LN, Luo Y, Wayne MJ, Graff EC, Ahmed B, O'Neill AM, Greene MW. Restricted feeding for 9 h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism 82: 1–13, 2018. doi: 10.1016/j.metabol.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 75. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27: 1212–1221.e3, 2018. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chaix A, Deota S, Bhardwaj R, Lin T, Panda S. Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell Rep 36: 109543, 2021. doi: 10.1016/j.celrep.2021.109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, Hackney KJ. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep 9: e14868, 2021. doi: 10.14814/phy2.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roh E, Choi KM. Health consequences of sarcopenic obesity: a narrative review. Front Endocrinol (Lausanne) 11: 332, 2020. doi: 10.3389/fendo.2020.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, Pahor M. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients 11: 1500, 2019. doi: 10.3390/nu11071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jones R, Pabla P, Mallinson J, Nixon A, Taylor T, Bennett A, Tsintzas K. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am J Clin Nutr 112: 1015–1028, 2020. doi: 10.1093/ajcn/nqaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, Harindhanavudhi T, Nair KS, Panda S, Mashek DG. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring) 28: 860–869, 2020. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr 110: 2108–2113, 2013. doi: 10.1017/S0007114513001359. [DOI] [PubMed] [Google Scholar]
- 83. Chausse B, Solon C, Caldeira da Silva CC, Masselli dos Reis IG, Manchado-Gobatto FB, Gobatto CA, Velloso LA, Kowaltowski AJ. Intermittent fasting induces hypothalamic modifications resulting in low feeding efficiency, low body mass and overeating. Endocrinology 155: 2456–2466, 2014. doi: 10.1210/en.2013-2057. [DOI] [PubMed] [Google Scholar]
- 84. Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. Meal timing regulates the human circadian system. Curr Biol 27: 1768–1775.e3, 2017. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 4: 345–353, 2018. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring) 27: 1244–1254, 2019. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Oliveira L da C, Morais GP, Ropelle ER, de Moura LP, Cintra DE, Pauli JR, de Freitas EC, Rorato R, da Silva ASR. Using intermittent fasting as a non-pharmacological strategy to alleviate obesity-induced hypothalamic molecular pathway disruption. Front Nutr 9: 858320, 2022. doi: 10.3389/fnut.2022.858320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nakandakari SCBR, Muñoz VR, Kuga GK, Gaspar RC, Sant'Ana MR, Pavan ICB, da Silva LGS, Morelli AP, Simabuco FM, da Silva ASR, de Moura LP, Ropelle ER, Cintra DE, Pauli JR. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav Immun 79: 284–293, 2019. doi: 10.1016/j.bbi.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 89. Brunetta HS, Politis-Barber V, Petrick HL, Dennis KMJH, Kirsh AJ, Barbeau PA, Nunes EA, Holloway GP. Nitrate attenuates high fat diet-induced glucose intolerance in association with reduced epididymal adipose tissue inflammation and mitochondrial reactive oxygen species emission. J Physiol 598: 3357–3371, 2020. doi: 10.1113/JP279455. [DOI] [PubMed] [Google Scholar]
- 90. da Cruz Rodrigues KC, Martins Pereira R, Peruca GF, Torres Barbosa LW, Ramos Sant’Ana M, Rosetto Muñoz V, Morelli AP, Moreira Simabuco F, Sanchez Ramos da Silva A, Esper Cintra D, Rochete Ropelle E, Pauli JR, de Moura LP. Short-term strength exercise reduces hepatic insulin resistance in obese mice by reducing PTP1B content, regardless of changes in body weight. Int J Mol Sci 22: 6402, 2021. doi: 10.3390/ijms22126402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pereira RM, da Cruz Rodrigues KC, Sant'Ana MR, da Rocha AL, Morelli AP, Veras ASC, Gaspar RS, da Costa Fernandes CJ, Teixeira GR, Simabuco FM, da Silva ASR, Cintra DE, Ropelle ER, Pauli JR, de Moura LP. FOXO1 is downregulated in obese mice subjected to short-term strength training. J Cell Physiol 237: 4262–4274, 2022. doi: 10.1002/jcp.30882. [DOI] [PubMed] [Google Scholar]
- 92. Kyriazis ID, Vassi E, Alvanou M, Angelakis C, Skaperda Z, Tekos F, Garikipati VNS, Spandidos DA, Kouretas D. The impact of diet upon mitochondrial physiology (Review). Int J Mol Med 50: 1–26, 2022. doi: 10.3892/ijmm.2022.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Edmunds LR, Xie B, Mills AM, Huckestein BR, Undamatla R, Murali A, Pangburn MM, Martin J, Sipula I, Kaufman BA, Scott I, Jurczak MJ. Liver-specific Prkn knockout mice are more susceptible to diet-induced hepatic steatosis and insulin resistance. Mol Metab 41: 101051, 2020. doi: 10.1016/j.molmet.2020.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao Q-Y, Ge L-H, Zhang K, Chen H-F, Zhan X-X, Yang Y, Dang Q-L, Zheng Y, Zhou H-B, Lyu J-X, Fang H-Z. Assessment of mitochondrial function in metabolic dysfunction-associated fatty liver disease using obese mouse models. Zool Res 41: 539–551, 2020. doi: 10.24272/j.issn.2095-8137.2020.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21: 739–746, 2015. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 96. Chausse B, Vieira-Lara MA, Sanchez AB, Medeiros MHG, Kowaltowski AJ. Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS One 10: e0120413, 2015. doi: 10.1371/journal.pone.0120413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Beleza J, Rizo-Roca D, Ascensão A, Magalhães J. Targeting mitochondria with sweat: improving mitochondrial function with physical activity. In: Mitochondrial Biology and Experimental Therapeutics. Cham, Switzerland: Springer International Publishing, 2018, p. 379–406. doi: 10.1007/978-3-319-73344-9_18. [DOI] [Google Scholar]
- 98. Fletcher JA, Linden MA, Sheldon RD, Meers GM, Morris EM, Butterfield A, Perfield JW, Thyfault JP, Rector RS. Fibroblast growth factor 21 and exercise-induced hepatic mitochondrial adaptations. Am J Physiol Gastrointest Liver Physiol 310: G832–G843, 2016. doi: 10.1152/ajpgi.00355.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gonçalves IO, Maciel E, Passos E, Torrella JR, Rizo D, Viscor G, Rocha-Rodrigues S, Santos-Alves E, Domingues MR, Oliveira PJ, Ascensão A, Magalhães J. Exercise alters liver mitochondria phospholipidomic profile and mitochondrial activity in non-alcoholic steatohepatitis. Int J Biochem Cell Biol 54: 163–173, 2014. doi: 10.1016/j.biocel.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 100. Gonçalves IO, Passos E, Rocha-Rodrigues S, Diogo CV, Torrella JR, Rizo D, Viscor G, Santos-Alves E, Marques-Aleixo I, Oliveira PJ, Ascensão A, Magalhães J. Physical exercise prevents and mitigates non-alcoholic steatohepatitis-induced liver mitochondrial structural and bioenergetics impairments. Mitochondrion 15: 40–51, 2014. doi: 10.1016/j.mito.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 101. Rosa-Caldwell ME, Lee DE, Brown JL, Brown LA, Perry RA, Greene ES, Carvallo Chaigneau FR, Washington TA, Greene NP. Moderate physical activity promotes basal hepatic autophagy in diet-induced obese mice. Appl Physiol Nutr Metab 42: 148–156, 2017. doi: 10.1139/apnm-2016-0280. [DOI] [PubMed] [Google Scholar]
- 102. Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS. Impact of various exercise modalities on hepatic mitochondrial function. Med Sci Sports Exerc 46: 1089–1097, 2014. doi: 10.1249/MSS.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Domingos MM, Rodrigues MFC, Stotzer US, Bertucci DR, Souza MVC, Marine DA, Gatto CDV, de Araújo HSS, de Andrade Perez SE. Resistance training restores the gene expression of molecules related to fat oxidation and lipogenesis in the liver of ovariectomized rats. Eur J Appl Physiol 112: 1437–1444, 2012. doi: 10.1007/s00421-011-2098-6. [DOI] [PubMed] [Google Scholar]
- 105. Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 13: 458–465, 2017. doi: 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93: 993–1017, 2013. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 107. Stanford KI, Middelbeek RJW, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 64: 2361–2368, 2015. [Erratum in Diabetes 64: 3334, 2015]. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Muñoz VR, Gaspar RC, Severino MB, Macêdo APA, Simabuco FM, Ropelle ER, Cintra DE, da Silva ASR, Kim YB, Pauli JR. Exercise counterbalances Rho/ROCK2 signaling impairment in the skeletal muscle and ameliorates insulin sensitivity in obese mice. Front Immunol 12: 702025, 2021. doi: 10.3389/fimmu.2021.702025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.