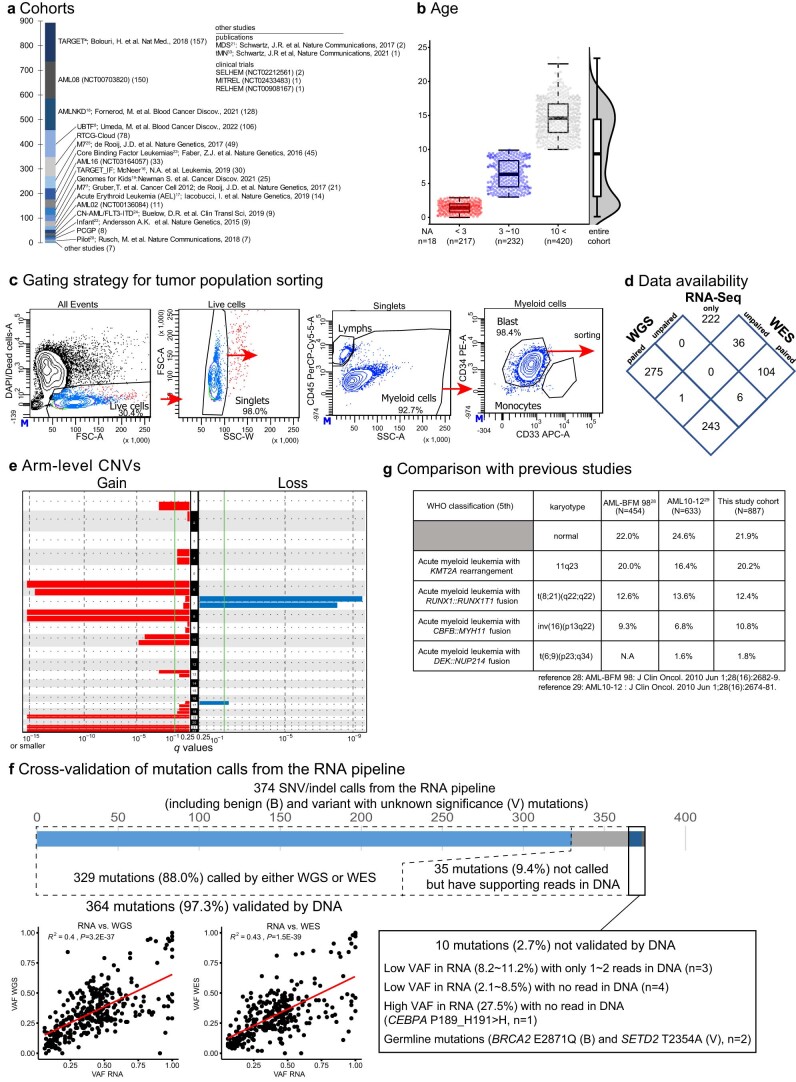
Extended Data Fig. 1. Cohort details.
a. Data source of each patient with acute myeloid leukemia (AML), including publications and clinical trials. b. Age distribution of patients at diagnosis (red: age<3, blue: 3<age<10, gray: 10<age). Lines of the box represent 25% quantile, median, and 75% quantile. The upper whisker represents the higher value of maxima or 1.5 x interquartile range (IQR), and the lower whisker represents the lower value of minima or 1.5 x IQR. NA: not available. c. Representative gating strategy for sorting of the myeloid cell population. Vertical and horizontal axes are linear for FSC (forward scatter) and SSC (side scatter) and log-scaled for fluorescence-conjugated antibodies. CD34 gating was adjusted for individual patients depending on the positivity. d. A Venn diagram showing data platforms available for each patient. WGS: whole-genome sequencing, WES: whole-exome sequencing, RNA-Seq: RNA-sequencing. e. Results of GISTIC (Genomic Identification of Significant Targets in Cancer) analysis for arm-level chromosomal events. The left panel shows the enrichment of chromosomal gains (red), and the right panel shows the enrichment of chromosomal losses (blue). Green lines show a significance threshold for q values (0.25). f. Cross-validation of single nucleotide variant (SNV) and insertion/deletion (indel) calls from the RNA pipeline using whole-genome/exome sequencing (WGS/WES) data. The bar graph shows mutation calls and the validation status. For those also called from DNA data, a comparison of variant allele frequency (VAF) and Pearson’s correlation are shown in the bottom left, and the statistical test was performed as two-sided. A regression line is shown in red. For unvalidated calls, details are shown in the bottom right. g. A comparison of major classes of the World Health Organization (WHO) classification in the study cohort with karyotyping in previous large pediatric AML cohorts.