Abstract
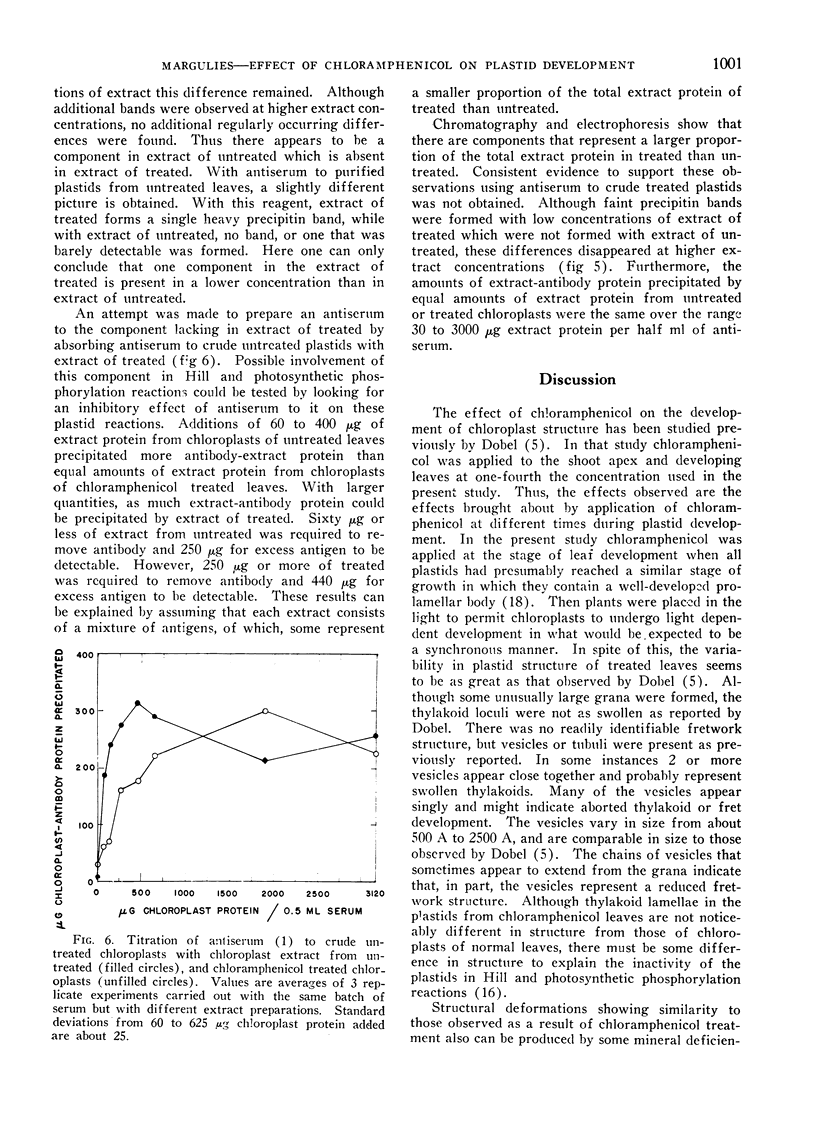
Greening of leaves of Phaseolus vulgaris in the presence of chloramphenicol inhibits formation of A) total chloroplast protein, B) an easily extractable fraction removed during isolation of chloroplasts in isotonic media by differential centrifugation, and C) the insoluble lamellar fraction which remains after extracting osmotically shocked freeze-dried plastids. The inhibition of insoluble chloroplast protein formation is correlated with decreased formation of lamellae and increased formation of vesicular structures. In contrast, chloramphenicol increases the formation of a fraction not removed during differential centrifugation, but removed by water extraction after osmotic shock and freeze-drying of chloroplasts. Analysis of this fraction by electrophoresis and column chromatography, indicates that the increased accumulation of this protein fraction is largely due to accumulation of a protein which is normally present in this fraction in small quantities. It was suggested that this protein may be a precursor which is normally incorporated into the lamellae. The protein extracted from freeze-dried chloroplasts of chloramphenicol treated chloroplasts contains a smaller proportion of one or more proteins than a similar extract of untreated plastids. However, per plastid, no such difference exists.
Full text
PDF



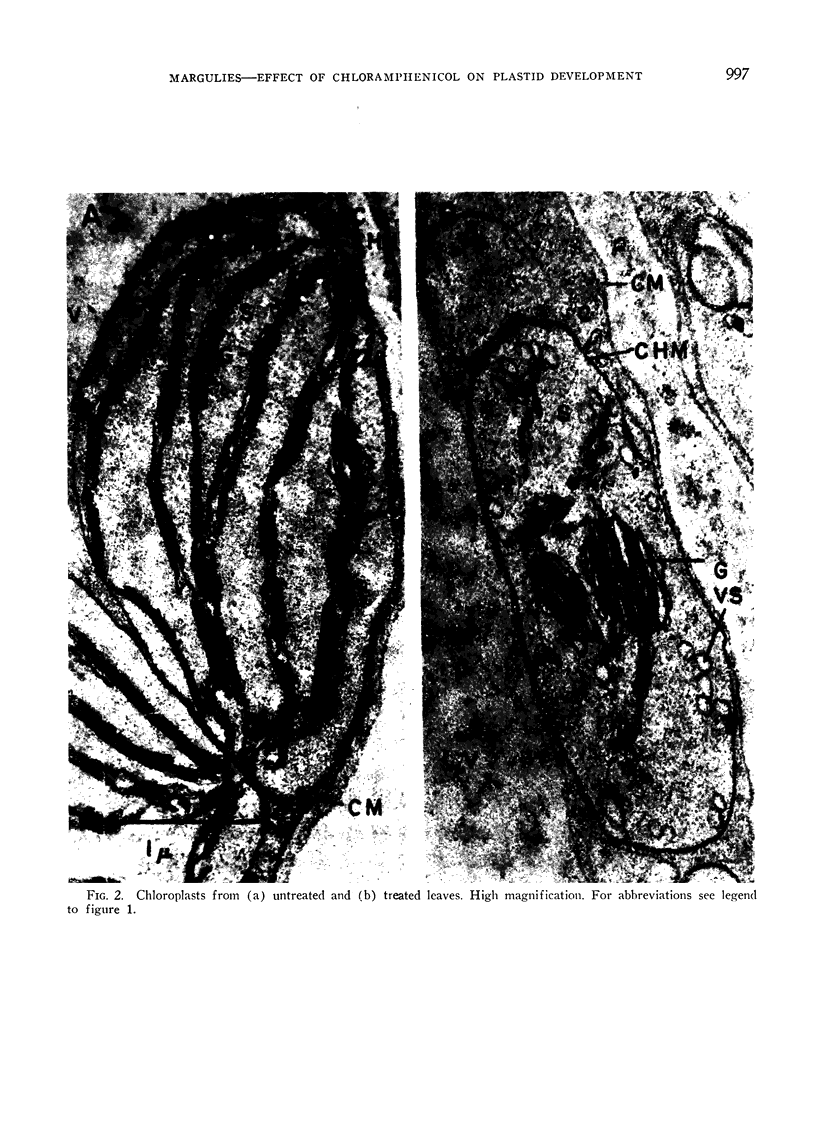
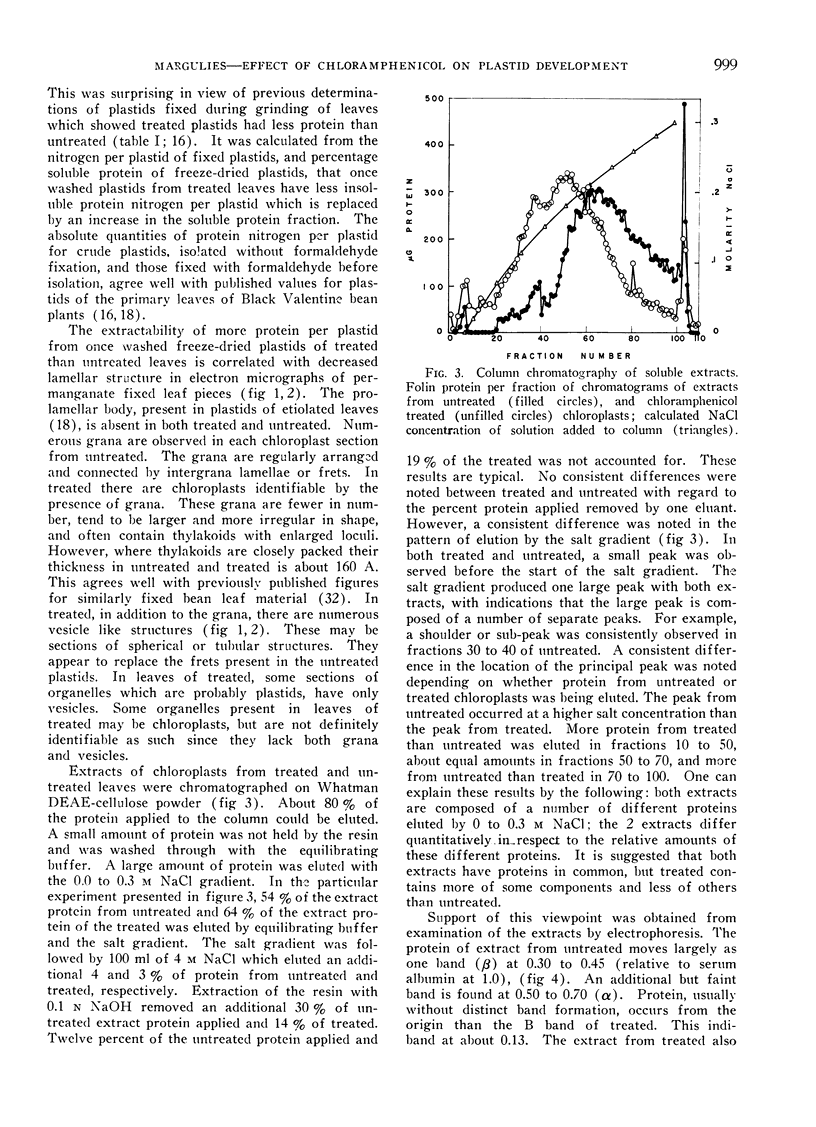
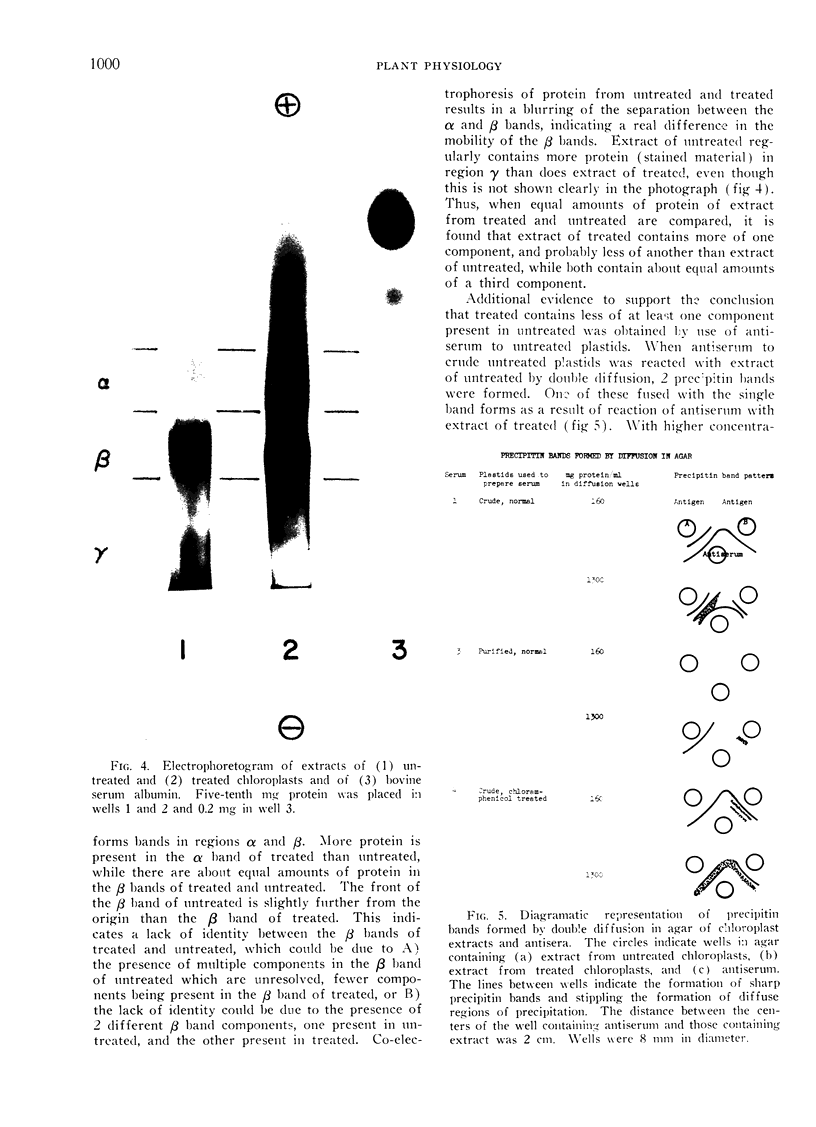


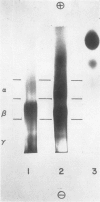
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis S. C., Schiff J. A., Epstein H. T. Studies of chloroplast development in Euglena. 9. Chloroplast antigens and their appearance during chloroplast development. J Protozool. 1965 May;12(2):281–290. doi: 10.1111/j.1550-7408.1965.tb01853.x. [DOI] [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light Dependent Development of Seedlings of Phaseolus vulgaris var. Black Valentine, With Particular Reference to Development of Photosynthetic Activity. Plant Physiol. 1962 Jul;37(4):473–480. doi: 10.1104/pp.37.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Relationship Between Red Light Mediated Glyceraldehyde-3-Phosphate Dehydrogenase Formation and Light Dependent Development of Photosynthesis. Plant Physiol. 1965 Jan;40(1):57–61. doi: 10.1104/pp.40.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK R. B., PON N. G. Correlation of structure with function in Spinacea oleracea chloroplasts. J Mol Biol. 1961 Feb;3:1–10. doi: 10.1016/s0022-2836(61)80002-8. [DOI] [PubMed] [Google Scholar]
- PIERPOINT W. S. Mitochondrial preparations from the leaves of tobacco (Nicotiana tabacum). 4. Separation of some components by density-gradient centrifuging. Biochem J. 1962 Jan;82:143–148. doi: 10.1042/bj0820143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGO B. G., POGO A. O. INHIBITION BY CHLORAMPHENICOL OF CHLOROPHYLL AND PROTEIN SYNTHESIS AND GROWTH IN EUGLENA GRACILIS. J Protozool. 1965 Feb;12:96–100. doi: 10.1111/j.1550-7408.1965.tb01820.x. [DOI] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]
- WEIER T. E., STOCKING R., BRACKER C. E., RISLEY E. B. THE STRUCTURAL RELATIONSHIPS OF THE INTERNAL MEMBRANE SYSTEMS OF IN SITU AND ISOLATED CHLOROPLASTS OF HORDEUM VULGARE. Am J Bot. 1965 Apr;52:339–352. [PubMed] [Google Scholar]
- Weier T. E., Engelbreicht A. H., Harrison A., Risley E. B. Subunits in the membranes of chloroplasts of Phaseolus vulgaris, Pisum sativum, and Aspidistra sp. J Ultrastruct Res. 1965 Aug;13(1):92–111. doi: 10.1016/s0022-5320(65)80091-0. [DOI] [PubMed] [Google Scholar]