Abstract
Lipid overload or metabolic stress has gained popularity in research that explores pathological mechanisms that may drive enhanced oxidative myocardial damage. Here, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06 to 1 mM) for either 4 or 24 h to study its potential physiological response to cardiac cells. Briefly, assays performed included metabolic activity, cholesterol content, mitochondrial respiration, and prominent markers of oxidative stress, as well as determining changes in mitochondrial potential, mitochondrial production of reactive oxygen species, and intracellular antioxidant levels like glutathione, glutathione peroxidase and superoxide dismutase. Cellular damage was probed using fluorescent stains, annexin V and propidium iodide. Our results indicated that prolonged exposure (24-hours) to palmitic acid doses ≥ 0.5 mM significantly impaired mitochondrial oxidative status, leading to enhanced mitochondrial membrane potential and increased mitochondrial ROS production. While palmitic acid dose of 1 mM appeared to induce prominent cardiomyoblasts damage, likely because of its capacity to increase cholesterol content/ lipid peroxidation and severely suppressing intracellular antioxidants. Interestingly, short-term (4-hours) exposure to palmitic acid, especially for lower doses (≤ 0.25 mM), could improve metabolic activity, mitochondrial function and protect against oxidative stress induced myocardial damage. Potentially suggesting that, depending on the dose consumed or duration of exposure, consumption of saturated fatty acids such as palmitic acid can differently affect the myocardium. However, these results are still preliminary, and in vivo research is required to understand the significance of maintaining intracellular antioxidants to protect against oxidative stress induced by lipid overload.
Keywords: Cardiac function, Dyslipidemia, Lipid overload, Palmitic acid, Mitochondrial function, Oxidative stress
Graphical Abstract
1. Introduction
Metabolic syndrome is a major pathological feature that is facilitated by overnutrition, especially through increased intake of saturated fatty acids, together with reduced physical activity [1]. Such metabolic abnormalities are driven by obesity and can cause other complications like type 2 diabetes (T2D) and cardiovascular diseases (CVD) [2]. In fact, enhanced free fatty acids (FFAs) tissue delivery, is known to cause myocardial injury within a metabolic syndrome [3], [4]. Mitochondria have become one of the primary targets being investigated for their role during the development of intramyocardial lipotoxicity [3], [5]. Lipids remain the main source of fuel within the mitochondria, however enhanced intake of FFAs can also hinder the efficiency of oxidative phosphorylation [6]. Thus, a clear understanding of the functional composition of these contradictory effects and their potential impact on mitochondrial-cellular energetics or redox status remains to be fully elucidated.
Like most cells, cardiac cells store FFAs as triacylglycerol and package them into cytoplasmic lipid droplets, while also remaining their predominant source of energy [6]. Emerging data indicates intramyocardial lipid excess can hinder the efficiency of the mitochondrial respiratory process, favouring an imbalance in redox status and further contributing to enhanced cellular damage [7], [8]. Apparently, the use of in vitro H9c2 cardiomyoblasts has increasingly become an experimental model to provide insights into cellular mechanisms and responses at a molecular level, making them invaluable for drug screening and mechanistic studies [9], [10]. Thus, while acknowledging that in vivo models have their place in understanding whole-body physiology, in vitro systems like H9c2 cells may offer a more focused and controlled approach to cardiac research.
Palmitic acid is one of the common saturated FFAs that are normally synthesized endogenously via de novo lipogenesis within the human body [11], however, it can also be obtained through diet [12]. The nutritional value of palmitic acid is quite controversial due to its putative detrimental health effects [13], which potentially shadows its multiple crucial physiological activities such as maintaining the cellular structure, and various cell signaling mechanisms [12], [14]. Therefore, there is a need to understand or decipher the pathophysiological role of palmitic acid within cardiac cells. Importantly, there is already evidence indicating that low doses of palmitic acid can be beneficial to liver cells by improving mitochondrial metabolism [15]. However, very limited information affirms these beneficial effects of low dose exposure to palmitic acid within the myocardium. In this study, we have evaluated and compared the dose and time-dependent effects of palmitic acid on metabolic activity, cholesterol content, mitochondrial respiration processes, oxidative status, and cellular damage in cultured H9c2 cardiomyoblasts.
2. Methods
2.1. Cell culture conditions for H9c2 cardiomyoblasts
The rat heart ventricular-derived H9c2 cardiomyoblasts were purchased from the American Type Culture Collection (Manassas, VA, USA; catalog number: CRL-1446). Briefly, H9c2 cardiomyoblasts were cultured in Dulbecco’s modified Eagle medium (Lonza, MD, USA) supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany) at standard tissue culture conditions (37 °C, in humidified air and 5% CO2). Cells were regularly sub-cultured at a confluency of 80–90% and seeded in 96-well or 6-well plates at a density of 5 × 104 cells/ml for all the assays performed. To test the dose and time-dependent effects of palmitic acid (Sigma–Aldrich St. Louis, MO, USA), H9c2 cardiomyoblasts were exposed to its different concentrations (0.06, 0.125, 0.25, 0.5, 0.75, and 1 mM) for either 4 or 24 h [16]. Our previous study has already reported on the potential toxic effects of exposing cultured cardiomyoblasts to chronic concentrations of palmitic acid over a period of 24 h [17]. This information, combined with other research [15] has provided a need to understand the impact of various doses of palmitate on cardiac cell survival, especially through the regulation of intracellular antioxidant mechanisms. Notably, to achieve solubility, palmitic acid was in prepared in 1% bovine serum albumin (Sigma–Aldrich St. Louis, MO, USA) using a modified method that has already been previously described by [18].
2.2. Determination of metabolic activity of H9c2 cardiomyoblasts
Cytoplasmic adenosine triphosphate (ATP) was quantified using a CellTiter-Glo® Luminescent Cell Viability Assay Kit from Promega (MA, USA), as per the manufacturer’s instructions. Luminescence emission, as a measurement of ATP production, was assessed using the SpectraMax i3x multi-mode microplate reader (Molecular Devices, CA, USA). Proteins were quantified for normalization using Bradford assay (Bio-Rad Laboratories, CA, USA).
2.3. Assessment of total cholesterol content and levels of lipid peroxidation of H9c2 cardiomyoblasts
To quantify cholesterol content, the Cholesterol/ Cholesteryl Ester Assay Kit from Abcam (Cambridge, UK) was used, as per manufacturer's instructions. The fluorescence (for the cholesterol assay) was measured at an excitation and emission of 535/587 nm. Lipid peroxidation was measured through the production of malondialdehyde (MDA), using an OxiSelect™ Thiobarbituric Acid Reactive Substances (TBARS) Assay Kit from Cell Biolabs (San Diego, USA), per the manufacturer’s instructions. SpectraMax i3x multi-mode microplate reader (Molecular Devices, CA, USA) was used to read the absorbance at 532 nm.
2.4. Assessment of mitochondrial respiration of H9c2 cardiomyoblasts
Mitochondrial respiration measured by oxygen consumption rate (OCR) and glycolytic energy measured by extracellular acidification rate (ECAR) were assessed using the Mito Stress Kit and XF-96 Extracellular Flux Analyser from Seahorse Bioscience (MA, USA), following a method that has already been described [19]. Briefly, OCR (pmol/min) and ECAR (mpH/min) were measured by injecting 1 μM oligomycin (ATP-synthase inhibitor), 0.75 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP, a mitochondrial uncoupler), and 5 μM rotenone (complex I inhibitor) plus antimycin A (complex III inhibitor) at specified time points. Similar mitochondrial activity measured by ATP production (Section 2.2), protein content was also used to normalize the OCR and ECAR data, as reported in absolute rates (pmol/min/mg protein and pmH/min/mg protein, respectively. All Seahorse reagents were bought from Agilent Technologies (Santa Clare, USA).
2.5. Evaluation of mitochondrial mass and ROS production, including changes in mitochondrial membrane potential (Δψm)
Mitochondrial ROS production, mitochondrial mass, and changes in Δψm were assessed by using fluorescent dyes, MitoSOX Red (Thermo Fisher Scientific, MA, USA), MitoTracker Green (Thermo Fisher Scientific, MA, USA), and 5,5′,6,6′-tetrachloro-1,1′,3,3-tetraethylbenzimidazolyl-carbocyanine iodide (JC-10; Sigma-Aldrich, St Louis, MO, USA), respectively, following the manufacturer’s instructions. Fluorescent measurements for mitochondrial ROS production and mitochondrial mass were measured using BD Accuri® C6 flow cytometer (Becton Dickinson, NJ, USA). Mitochondrial integrity was quantified by measuring the fluorescence intensity of JC-10 aggregates, orange fluorescence at ∼590 nm (excited by 540 nm), and JC-10 monomers, green fluorescence at ∼525 nm (excited by 490 nm), using the SpectraMax i3x multi-mode microplate reader.
2.6. Evaluation of apoptosis using annexin V and propidium iodide of H9c2 cardiomyoblasts
Annexin V-FITC from Invitrogen (Carlsbad, CA, USA), and propidium iodide from Sigma-Aldrich (St Louis, MO, USA) were used to detect early and late apoptosis as well as necrotic cells, respectively. Fluorescent measurements for both annexin V and propidium iodide were acquired using the BD Accuri C6 flow cytometer. The following channels were used, including the FITC signal detector FL1 (excitation= 488 nm; emission = 530 nm) for Annexin V positive cells, and the FL3 detector (excitation= 488 nm; emission = 670/LP) for propidium positive cells.
2.7. Evaluation of intracellular antioxidant capacity of H9c2 cardiomyoblasts
The levels of intracellular antioxidants, including total glutathione (GSH), glutathione peroxidase (GPx), and superoxide dismutase (SOD) were measured using an OxiSelect Total Glutathione Assay Kit from (Cell Bio-lab (San Diego, USA), Glutathione Peroxidase Assay Kit and the Superoxide Dismutase Activity Assay Kit from Abcam (Cambridge, UK), respectively, as per the manufacturer’s instructions. The relative optical density was measured using the SpectraMax i3x multi-mode microplate reader.
2.8. Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Results for all experiments were at least of three independent biological experiments, with each experiment containing at least three technical replicates. Statistical analysis was performed using GraphPad Prism software version 8.0.1 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between groups were performed using one-way multivariate ANOVA, followed by a Tukey post-hoc test, with p < 0.05 considered significant.
3. Results
3.1. Low doses and partial exposure to palmitic acid improves the metabolic activity of cardiomyoblasts
To understand the dose- and time-dependent effects of palmitic acid on metabolic activity, H9c2 cardiomyoblasts were exposed to different concentrations of palmitic acid for either 4 or 24 h before evaluation of ATP production. Results showed that palmitic acid induced a dose-dependent (0.06, 0.125, 0.25, 0.5, and 0.75 mM; p < 0.05 and p < 0.001, respectively) increase in metabolic activity of cells during the 4 h exposure (Fig. 1A). The rate of metabolic activity severely decreased in a dose dependent manner after 24 h exposure to palmitic acid (0.25, 0.5, 0.75 and 1 mM; p < 0.05 and p < 0.001), respectively (Fig. 1B).
Fig. 1.
The dose- and time-dependent effects of palmitic acid (4 and 24 h) treatment in ATP production in H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06, 0.125, 0.25, 0.75 and 1 mM) for 4 and 24 h followed by evaluation of metabolic activity using ATP kit. Results are represented as the mean ± standard deviation (SD) of three independent experiments, with at least six technical repeats, per experiment relative to the experimental control (Ctrl). *p < 0.05, * **p < 0.001 versus the experimental control.
3.2. Only high doses and prolonged exposure to palmitic acid increases cholesterol content and lipid peroxidation levels in cardiomyoblasts
We evaluated the dose and time-dependent effects of palmitic acid on cholesterol content and lipid peroxidation (MDA levels) in H9c2 cardiomyoblasts. The results showed that palmitic acid exposure only significantly reduced the cholesterol content at doses of 0.5 and 0.75 mM (p < 0.01 and p < 0.05, respectively) after treatment for 4 h (Fig. 2A). However, cholesterol levels were significantly increased at palmitic acid doses of 0.25, 0.5, 0.75 and 1 mM (p < 0.05, p < 0.01 and p < 0.001, respectively) after treatment for 24 h (Fig. 2B). In terms of lipid peroxidation, exposure to palmitic acid doses did not have any impact on MDA levels when treated for 4 h (Fig. 2C), but markedly increased MDA levels at a dose of 1 mM (p < 0.001) after treatment for 24 h (Fig. 2D).
Fig. 2.
The dose- and time-dependent effects of palmitic acid on total cholesterol content and lipid peroxidation in cultured H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various palmitic acid doses (0.06, 0.125, 0.25, 0.75 and 1 mM) for 4 and 24 h. Thereafter, total cholesterol content and malondialdehyde (MDA) levels as a measure of lipid oxidation were done. Results are expressed as the mean ± standard deviation (SD) of three independent experiments, with at least three technical repeats, relative to the experimental control (Ctrl). *p < 0.05, * *p < 0.01 * **p < 0.001 versus the experimental control.
3.3. Low doses and partial exposure to palmitic acid improves mitochondrial respiration and glycolytic energy levels in cardiomyoblasts
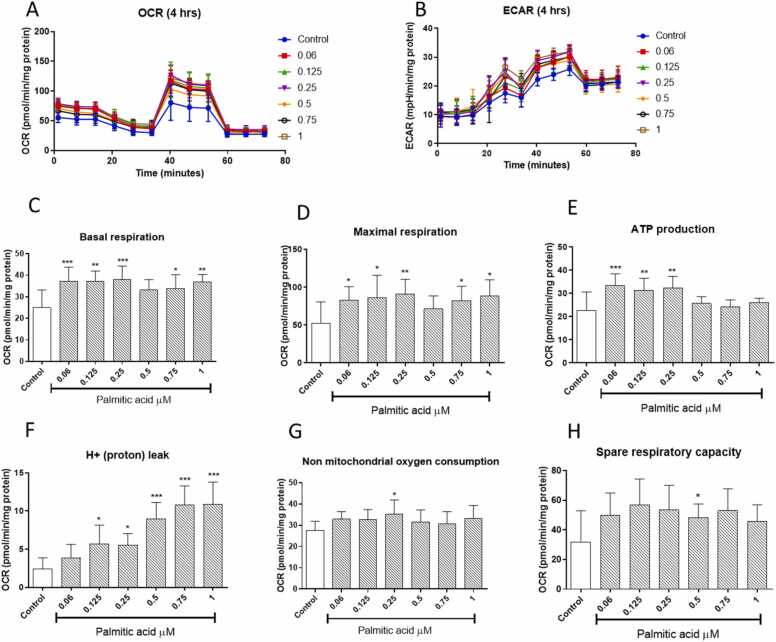
Next, we investigated the dose and time-dependent effects of palmitic acid on the mitochondrial OCR and extracellular acidification rate (ECAR), initially for treatment of 4 h (Fig. 3A and B). Our data demonstrated 4 h of palmitic acid was enough to increase oxygen consumption rate and glycolytic energy in all concentration tested as shown by increased OCAR and ECAR (Fig. 3A and B), respectively. Interestingly, almost all doses for palmitic acid improved the basal and maximal respiration after treatment for 4 h (Fig. 3C and D). Only the lower doses of palmitic acid of 0.06, 0.25 and 0.5 mM could improve ATP production after treatment for 4 h (Fig. 3E). However, most doses of palmitic acid did not markedly affect non-mitochondrial respiration or spare respiratory capacity (Fig. 3G and H).
Fig. 3.
The dose- and time-dependent effects of palmitic acid on mitochondrial respiration and glycolytic energy levels in cultured H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06, 0.125, 0.25, 0.75 and 1 mM) for 4 h. The graphs show oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) for all treatments (A and B). With others depicting basal respiration (B), maximal respiration (C), ATP production (E), proton leak (F), non-mitochondrial respiration (G), and spare respiratory capacity (H), respectively. Results are expressed as the mean ± standard deviation (SD) of at least three independent experiments, with at least six repeats per experiment, relative to the experimental control. *p < 0.05, * *p < 0.01, * **p < 0.001 versus the experimental control.
The dose- and time-dependent effects of palmitic acid on the mitochondrial OCR and ECAR were also evaluated after treatment of 24 h (Fig. 4A and B). Palmitic acid doses ≥ 0.5 Mm were able to reduce glycolytic energy (ECAR). Moreover, exposure to different concentrations of palmitic acid (especially starting from 0.25 to 1 mM) significantly suppressed the measured parameters, including basal and maximal respiration, as well as ATP production, non-mitochondrial respiration, and spare capacity after treatment for 24 h (Fig. 4 C, D, E, G and H). Interestingly, only doses of palmitic acid 0.75 and 1 mM reduced the proton leak, while that of 0.5 mM increased proton leak after treatment for 24 h (Fig. 4F). Even though lower concentrations (<02.5 mM) of palmitic acid did not markedly affect glycolytic energy (ECAR), the higher doses (≥0.5 mM) were associated with a significant reduction after treatment for 24 h (Fig. 4).
Fig. 4.
The dose and time dependent effects of treatment with palmitic acid for 24 h on mitochondrial respiration and glycolytic energy levels in cultured H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06, 0.125, 0.25, 0.75 and 1 mM) for 24 h. The graphs show oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) for all treatments (A and B). With others depicting basal respiration (B), maximal respiration (C), ATP production (E), proton leak (F), non-mitochondrial respiration (G), and spare respiratory capacity (H), respectively. Results are expressed as the mean ± standard deviation (SD) of at least three independent experiments, with at least six repeats, relative to the experimental control. *p < 0.05, * **p < 0.001 versus the experimental control.
3.4. Only high doses and prolonged exposure to palmitic acid increased mitochondrial ROS production and mitochondrial mass, while affecting membrane potential (ΔΨm) in cardiomyoblasts
We further evaluated the dose and time-dependent effects of palmitic acid on mitochondrial ROS production, including mitochondrial mass and changes in mitochondrial membrane potential (ΔΨm) after 4 or 24 h-exposure (Fig. 5). Our data showed that only palmitic acid concentrations of 0.75 and 1 mM (p < 0.01 and p < 0.001, respectively) for 4 h exposure, as well as 0.25 (p < 0.01) or ≥ 0.5 mM (p < 0.001) for 24 h could significantly increase mitochondrial ROS, respectively (Fig. 5A and B). Furthermore, dose and time dependent effects of palmitic acid on mitochondrial mass were done (Fig. 5C and D). With the results showing that palmitic acid does not affect mitochondrial mass after 4-hours treatment, but significantly increases this component at doses of ≥ 0.25 mM (at least p < 0.05) after 24-hours treatment (Fig. 5C and D). Alternatively, changes Δψm (red/green fluorescence) or rather mitochondrial membrane potential was significantly increased after exposure to palmitic acid at doses of 0.125 and 0.25 mM (p < 0.05 and p < 0.001, respectively) after 4 h, also showing a similar trend for 24-hour treatment (Fig. 5E and F). Then mitochondrial membrane potential was significantly reduced for palmitic acid of 0.5 to 1 mM (p < 0.001) for both 4 and 24 h (Fig. 5E and F).
Fig. 5.
The dose and time-dependent effects of palmitic acid on production of mitochondrial reactive oxygen species (ROS), mitochondrial mass, and changes in mitochondrial membrane potential (ΔΨm) in cultured H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06, 0.125, 0.25, 0.75 and 1 mM) for 4 and 24 h. MitoSox fluorescent stain was used for detection of mitochondrial ROS production. MitoTracker Green was used for mitochondrial mass. Fluorescent measurements for mitochondrial integrity included quantification of intensity for JC-10 aggregates, orange fluorescence at ∼590 nm (excited by 540 nm), and JC-10 monomers, green fluorescence at ∼525 nm (excited by 490 nm). Results are expressed as the mean ± standard deviation (SD) of three independent experiments, with at least six technical repeats, relative to the experimental control (Ctrl). *p < 0.01, * *p < 0.01, * **p < 0.001 versus experimental control.
3.5. Only high doses and prolonged exposure to palmitic acid reduced intracellular antioxidant levels in cardiomyoblasts
We also evaluated the dose and time-dependent effects of palmitic acid on intracellular antioxidant enzymes, including GSH content, GPx, and SOD (Fig. 6). The results showed that palmitic acid doses did not significantly affect total GSH content after 4 h exposure, but markedly decreased the total GSH content at doses of 0.75 and 1 mM (p < 0.001) after treatment for 24 h (Fig. 6A and B). The GPx levels were also not significantly affected by palmitic acid doses after 4 h exposure, but all doses markedly reduced the GPx levels (at least p < 0.05) following treatment for 24 h (Fig. 6C and D). Similar effects were seen with SOD activity, with only the highest dose of palmitic acid (1 mM) showing significance in reducing SOD levels (p < 0.05) after 24 h exposure (Fig. 6E and F).
Fig. 6.
The dose and time-dependent effect of palmitic acid on intracellular antioxidant levels, including glutathione (GSH) content, glutathione peroxidase (GPx), and superoxide dismutase (SOD) in cultured H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06, 0.125, 0.25, 0.75 and 1 mM) for 4 and 24 h. Results are expressed as the mean ± standard deviation (SD) of three independent experiments, with at least three technical repeats, relative to the experimental control (Ctrl). *p < 0.05, * *p < 0.01, * **p < 0.001 versus experimental control.
3.6. Only high doses and prolonged exposure to palmitic acid promoted cellular apoptosis in cardiomyoblasts
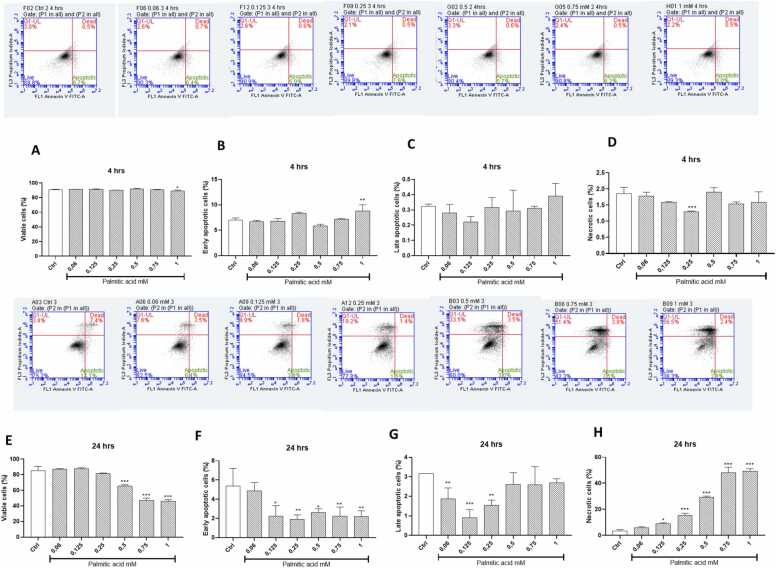
The dose and time-dependent effects of palmitic acid on the rate of early and late cellular apoptosis were also evaluated using annexin V and propidium iodide, respectively (Fig. 7). The results showed that all doses of palmitic acid did not affect the viability of cells after 4 h exposure, while the dose of 1 mM (p < 0.05) significantly increased early apoptotic rate, and the dose of 0.25 mM (p < 0.001) markedly reduced cellular necrotic rate (Fig. 7A to D). Interestingly, opposite effects were seen with higher palmitic acid doses (0.5, 0.75, and 1 mM) reducing cell viability (p < 0.001) and early/late apoptosis after 24-hours treatment, while significantly increasing the levels of cellular necrosis (p < 0.001) during the prolonged 24 h exposure (Fig. 7E to H).
Fig. 7.
The dose and time dependent effects of palmitic acid the rate of early and late cellular apoptosis in cultured H9c2 cardiomyoblasts. Briefly, H9c2 cardiomyoblasts were exposed to various doses of palmitic acid (0.06, 0.125, 0.25, 0.75 and 1 mM) for 4 (A-D) and 24 (E-H) hours before fluorescent staining with annexin V and propidium iodide. Within each of the representative flow cytometry fluorescence dot plot images, the upper right quadrant illustrates cells showing a combination stain of annexin V and propidium iodide to indicate late apoptotic. Within the same image, cells on the lower right quadrant indicate early apoptosis. While those on the lower and upper left quadrant are viable are necrotic cells, respectively. The graphs depict viable cells (A), early apoptosis (B), late apoptosis (C), and necrosis (D). Results are expressed as the mean ± standard deviation (SD) of three independent experiments, with at least three technical repeats, relative to the experimental control (Ctrl). *p < 0.05, * *p < 0.01, * **p < 0.001 versus the experimental control.
4. Discussion
Lipid overload or consistent exposure to saturated levels of FFAs, such as palmitic acid, is a well-known mechanism that promotes the detrimental effects leading to myocardial toxicity [20], [21], [22]. In fact, lipid overload has been linked with impaired mitochondrial function, including altered respiratory process that potentially contributes to excessive production of toxic ROS, within various experimental models of metabolic disease [8], [23]. The current state of knowledge indicates that impaired lipid profiles, especially hypercholesterolemia is major driver of lipid peroxidation, which is connected to the development of myocardial dysfunction within conditions of T2D [24], [25]. Indeed, the current study showed that exposure of H9c2 cardiomyoblasts to elevated doses of palmitic acid (especially for the highest dose of 1 mM) was associated with a reduction in metabolic activity (ATP production) that was concomitant to enhanced cholesterol content and elevated lipid peroxidation products (MDA levels) after 24 h exposure in cultured cardiomyoblasts.
Although a healthy heart requires a relatively high demand of FFAs to maintain its optimal contractile function (translating to enhanced ATP production) [26], prolonged exposure to these saturated lipid molecules could lead to impairments in mitochondrial function [27] and enhanced generation of oxidative stress within a pathological state [28]. Our findings also indicated that high doses of palmitic acid (≥0.5 mM) could induce a dose-dependent suppression of mitochondrial respiratory capacity that was concurrent to reduced mitochondrial potential, proton leak, and enhanced generation of toxic ROS. It has been previously hypothesized that metabolic stress-associated changes are consistent with cellular processes that affect mitochondrial mass and increased oxidative-myocardial damage [29]. Interestingly, our results indicated that relatively high doses of palmitic acid (≥0.5 mM) could increase mitochondrial mass that was consistent with impairments in mitochondrial respiratory capacity and enhanced generation of ROS. In fact, our recent study already supports the notion that impaired substrate metabolism, through abnormally elevated levels of palmitate, may potentially drive the pathological features of oxidative cardiac damage [10], [17], [30]. Beyond its potential detrimental effects on cellular function of the development of mitochondrial dysregulation, in this study, we have investigated the dose and time-dependent effects of palmitic acid on intracellular antioxidant responses and oxidative damage. The major focus was on understanding whether low doses of palmitic acid (≤0.25 mM) could improve mitochondrial respiratory process, while enhancing intracellular antioxidants to neutralize the detrimental effects of toxic ROS and lipid peroxidation within these cultured cells.
It is still unclear whether an ideal physiological saturated fatty acids consumption level required for maintaining normal mitochondrial and cellular function exists. However, there are studies that have reported the potential benefits of lower levels intake of saturated fatty acids such as palmitic acid [15], [31]. Here, reducing the palmitic acid exposure to 4 h revealed favorable outcome to the metabolic activity of H9c2 cardiomyoblasts, this was shown by an increased ATP production which was concurrent with reduced cholesterol content at high doses of palmitic acid (≥0.5 mM), but no significant effect was observed with the lipid peroxidation (MDA levels). Unlike 24 h exposure, short term (4-hour) exposure to palmitic acid was able to enhance mitochondrial respiratory capacity, which was supported by increased maximal respiration, proton leak and spare capacity. These positive effects were associated with enhanced glycolytic energy capacity and non-mitochondrial respiration, perhaps suggesting that a substrate switch, especially favouring glucose utilization or reduced oxidation could be beneficial to the cardiomyoblasts. Interestingly, this hypothesis has been previously tested within some experimental studies showing that a fine balance in substrate utilization should be maintained for reducing myocardial abnormalities within an impaired metabolic state [10], [32]. With existing evidence further showing that activation of intracellular antioxidant mechanisms is necessary to protect against oxidative stress-induced damage within the heart [33], [34]. It has also been reported that increased ROS production is associated with reduced antioxidant content in cardiac cells, leading to imbalanced of redox status [33], [35], [36]. Even though high dose of palmitic acid (≥0.75 mM) was able to slightly increase ROS production after 4 h, this was more pronounced for 24-hour exposure, also correlating to reduced intracellular antioxidant defenses and enhanced cellular damage especially for a dose of 1 mM during this prolonged treatment period.
Others have already highlighted the significant role of enhanced ROS production in palmitate-induced apoptosis in H9c2 cells [37]. In this study, increased ROS production after 24 h exacerbated the rate of apoptosis, while this was not the case with 4-hour exposure to palmitic acid. Interestingly, it has already been indicated that low levels of saturated FFAs like palmitate can be beneficial to liver cells by boosting mitochondrial metabolism [15]. Excessive mitochondrial ROS generation, together with altered NADPH oxidase systems are some of the mechanisms that have been noted to drive oxidative stress-induced cardiac damage [38], [39]. Although not investigated in the current study, others have shown that suppressed expression of molecular targets like heat shock factor 1, together with GPx could be targeted by palmitic acid to disturb calcium homeostasis and induce cell death as well as lipid peroxidation in cultured cardiomyoblasts [40]. Pretreatment of adult rat cardiomyocytes is with well-known antioxidants like resveratrol can be cardioprotective through effective modulation of sarco-endoplasmic reticulum ATPase 2a [41]an enzyme that is essential in regulating calcium homeostasis. Although such detrimental effects are consistent with abnormal regulation of FFAs, others have indicated that lipids can be beneficial to the heart by inducing hypertrophy in human cardiomyocytes, while controlling calcium dynamics, including action potential upstroke velocity and oxidative capacity [42]. However, even so, implications on the dose and exposure period appear to be essential to predict the influence of FFAs cardiac health. appears to be an important aspect to considered within a pathological state. Overall, our findings support literature indicating that abundance or prolonged exposure to palmitic acid may exacerbate oxidative myocardial damage [37], [43], [44]. Hence advocating the interest in investigating therapies that can enhancing intracellular antioxidants to improve mitochondrial function, alleviate the toxic effects of ROS and protect against myocardial damage.
5. Conclusion and future perspectives
This study uncovers significant potential metabolic defects that are promoted by the dose-dependent effects of palmitic acid, especially through driving mitochondrial dysfunction and oxidative stress-induced myocardial damage. Importantly, our data provides a need to understand the potential benefits of low dose or partial exposure in controlling metabolic flexibility of cardiomyoblasts. The study is not without limitations, like acknowledging the drawbacks of using H9c2 cardiomyoblasts, mainly because of their proliferative and glycolytic in nature, as well potential coexisting with skeletal muscle features. We also acknowledge that addition of cardiomyocyte images showing morphological changes in response to different doses/exposure periods to palmitic acid could have added value to the report. Thus, besides validation of such findings using a reliable in vivo model, additional evidence is necessary to comprehensively understand the precise molecular mechanisms implicating the involvement of myocardial lipid accumulation or toxicity within a compromised metabolic state.
Funding statement
The work reported herein was made possible through the research funding received from the National Research Foundation (NRF) (Grant numbers: 117829 and 141929), awarded to P.V.D as well as NRF of South Africa Thuthuka Program Grant 128296 and NRF support for rated scientist 113674 awarded to S.E. Mazibuko-Mbeje. Research of Capacity Development under the Early Investigators Program from the South African National Treasury (funding number: HDID8682/MB2022/EIP052) and Baseline funding from the Biomedical Research and Innovation Platform of the South African Medical Research Council (SAMRC) as well as Northwest University are acknowledged. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC or the funders.
CRediT authorship contribution statement
Marcheggiani Fabio: Writing – review & editing. Orlando Patrick: Writing – review & editing. Silvestri Sonia: Writing – review & editing, Supervision. Mazibuko-mbeje Sithandiwe E: Writing – review & editing, Funding acquisition, Supervision. Mthembu Sinenhlanhla: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Dludla Phiwayinkosi: Conceptualization, Resources, Supervision, Writing – review & editing, Funding acquisition, Writing – original draft. Tiano Luca: Writing – review & editing. Muller Christo JF: Supervision, Writing – review & editing. Nkambule Bongani: Supervision, Writing – review & editing. Cirilli Ilenia: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
S.X.H.M. is funded by the SAMRC through its Division of Research Capacity Development under the internship scholarship program from funding received from the South African National Treasury. Grant holders acknowledge that opinions, findings and conclusions or recommendations expressed in any publication generated by the SAMRC supported research are those of the authors, and that the SAMRC accepts no liability whatsoever in this regard.
Handling Editor: Prof. L.H. Lash
Data availability
All data used to support the findings of this study are included within the article. Raw data can be available on request after publication.
References
- 1.Esmailnasab N., Moradi G., Delaveri A. Risk factors of non-communicable diseases and metabolic syndrome. Iran. J. Public Health. 2012;41(7):77–85. [PMC free article] [PubMed] [Google Scholar]
- 2.Schaffer J.E. Lipotoxicity: when tissues overeat. Curr. Opin. Lipido. 2003;14(3):281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Fillmore N., Mori J., Lopaschuk G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014;171(8):2080–2090. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopaschuk G.D., et al. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 5.Kakimoto P.A., et al. Increased glycolysis is an early consequence of palmitate lipotoxicity mediated by redox signaling. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aon M.A., Bhatt N., Cortassa S.C. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg I.J., Trent C.M., Schulze P.C. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15(6):805–812. doi: 10.1016/j.cmet.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z., et al. Mechanisms of myocardial damage due to hyperlipidemia: a review of recent studies. Med Sci. Monit. 2022;28 doi: 10.12659/MSM.937051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins S.J., Borthwick G.M., Arthur H.M. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. Vitr. Cell Dev. Biol. Anim. 2011;47(2):125–131. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 10.Johnson R., et al. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016;60(4):922–934. doi: 10.1002/mnfr.201500656. [DOI] [PubMed] [Google Scholar]
- 11.Mashima T., Seimiya H., Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer. 2009;100(9):1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carta G., et al. Palmitic acid: physiological role, metabolism and nutritional implications. Front. Physiol. 2017;8:902. doi: 10.3389/fphys.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murru E., et al. Impact of dietary palmitic acid on lipid metabolism. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.861664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks F.M., et al. Dietary fats and cardiovascular disease: a presidential advisory from the american heart association. Circulation. 2017;136(3):e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 15.Liu L., et al. Low-level saturated fatty acid palmitate benefits liver cells by boosting mitochondrial metabolism via CDK1-SIRT3-CPT2 cascade. Dev. Cell. 2020;52(2):196–209.e9. doi: 10.1016/j.devcel.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Özlem Sultan A. In: Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. Genotoxicity, Marcelo L.L., Sonia S., editors. IntechOpen; Rijeka: 2017. p. Ch. 1. [Google Scholar]
- 17.Dludla P.V., et al. Palmitate-induced toxicity is associated with impaired mitochondrial respiration and accelerated oxidative stress in cultured cardiomyocytes: the critical role of coenzyme Q(9/10) Toxicol. Vitr. 2020;68 doi: 10.1016/j.tiv.2020.104948. [DOI] [PubMed] [Google Scholar]
- 18.Mazibuko S.E., et al. Amelioration of palmitate-induced insulin resistance in C₂C₁₂ muscle cells by rooibos (Aspalathus linearis) Phytomedicine. 2013;20(10):813–819. doi: 10.1016/j.phymed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Mazibuko-Mbeje S.E., et al. Antimycin A-induced mitochondrial dysfunction is consistent with impaired insulin signaling in cultured skeletal muscle cells. Toxicol. Vitr. 2021;76 doi: 10.1016/j.tiv.2021.105224. [DOI] [PubMed] [Google Scholar]
- 20.van de Weijer T., Schrauwen-Hinderling V.B., Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92(1):10–18. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 21.Schulze P.C. Myocardial lipid accumulation and lipotoxicity in heart failure. J. Lipid Res. 2009;50(11):2137–2138. doi: 10.1194/jlr.R001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wende A.R., Abel E.D. Lipotoxicity in the heart. Biochim Biophys. Acta. 2010;1801(3):311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubin A.M., et al. Role of reactive oxygen species in the regulation of cardiac contractility. J. Mol. Cell Cardiol. 2011;50(5):884–893. doi: 10.1016/j.yjmcc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Shabalala S.C., et al. Detrimental effects of lipid peroxidation in type 2 diabetes: exploring the neutralizing influence of antioxidants. Antioxid. (Basel) 2022;11(10) doi: 10.3390/antiox11102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Lleó A.M., et al. Diabetes and familial hypercholesterolemia: interplay between lipid and glucose metabolism. Nutrients. 2022;14(7) doi: 10.3390/nu14071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolwicz S.C., Purohit Jr, S., Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013;113(5):603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisneski J.A., et al. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J. Clin. Invest. 1987;79(2):359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faria A., Persaud S.J. Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol. Ther. 2017;172:50–62. doi: 10.1016/j.pharmthera.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Bugger H., Abel E.D. Mitochondria in the diabetic heart. Cardiovasc Res. 2010;88(2):229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dludla P.V., et al. N-Acetyl cysteine ameliorates hyperglycemia-induced cardiomyocyte toxicity by improving mitochondrial energetics and enhancing endogenous Coenzyme Q(9/10) levels. Toxicol. Rep. 2019;6:1240–1245. doi: 10.1016/j.toxrep.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imamura F., et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med. 2017;14(10) doi: 10.1371/journal.pmed.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wende A.R., et al. Maintaining myocardial glucose utilization in diabetic cardiomyopathy accelerates mitochondrial dysfunction. Diabetes. 2020;69(10):2094–2111. doi: 10.2337/db19-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Geest B., Mishra M. Role of oxidative stress in diabetic cardiomyopathy. Antioxidants. 2022;11(4) doi: 10.3390/antiox11040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dludla P.V., et al. Aspalathin protects the heart against Hyperglycemia-induced oxidative damage by up-regulating Nrf2 expression. Molecules. 2017;22(1) doi: 10.3390/molecules22010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013;2013 doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol. Sci. 2011;32(3):125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Wei C.D., et al. Palmitate induces H9c2 cell apoptosis by increasing reactive oxygen species generation and activation of the ERK1/2 signaling pathway. Mol. Med. Rep. 2013;7(3):855–861. doi: 10.3892/mmr.2013.1276. [DOI] [PubMed] [Google Scholar]
- 38.Dludla P.V., et al. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr. Metab. 2017;14:45. doi: 10.1186/s12986-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y., et al. Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by N‑acetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress. Cell Death Dis. 2018;9(5):568. doi: 10.1038/s41419-018-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C.D., et al. [Literature research and traditional Chinese medicine properties of Aspalathus linearis] Zhongguo Zhong Yao Za Zhi. 2021;46(8):1960–1966. doi: 10.19540/j.cnki.cjcmm.20200915.410. [DOI] [PubMed] [Google Scholar]
- 41.Louis X.L., et al. Resveratrol prevents palmitic-acid-induced cardiomyocyte contractile impairment. 5th Eur. Sect. Meet. Int. Acad. Cardiovasc. Sci. 2019;01(01):1132–1140. doi: 10.1139/cjpp-2019-0051. [DOI] [PubMed] [Google Scholar]
- 42.Yang X., et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2019;13(4):657–668. doi: 10.1016/j.stemcr.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ly L.D., et al. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017;49(2) doi: 10.1038/emm.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L., et al. Increased fatty acid metabolism attenuates cardiac resistance to β-adrenoceptor activation via mitochondrial reactive oxygen species: a potential mechanism of hypoglycemia-induced myocardial injury in diabetes. Redox Biol. 2022;52 doi: 10.1016/j.redox.2022.102320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are included within the article. Raw data can be available on request after publication.