Abstract
Immune checkpoint inhibitors (ICIs) have become a cornerstone of treatment for many solid organ malignancies. Alongside increasing use, the occurrence of immune-related adverse events (irAEs) has also increased and remains a significant challenge when treating patients with ICI. The underlying pathophysiology of irAE development for many organ systems is yet to be elucidated, but may involve unmasking of latent autoimmunity, increased T-cell recognition of shared antigens on cancer and normal tissue and ICI-triggered immune dysregulation with overactivation of proinflammatory pathways and suppression of immune control pathways. Management strategies for irAEs have historically been borrowed from paradigms for conventional autoimmune conditions such as inflammatory bowel disease and autoimmune hepatitis; however, recent translational efforts have clearly demonstrated key differences in underlying immune signalling pathways. As we begin to understand these differences, we must adapt a more targeted approach to immunosuppression and exercise a more nuanced approach with the multiple biologic agents available to mitigate ICI-related toxicity without reversing the antitumour effect of ICI. In this review, we focus on three key immune-related toxicities where recent clinical and translational work has provided nuanced insights into pathogenesis and treatment strategies: enterocolitis, hepatitis and cardiovascular toxicity including myocarditis.
Key words: immunotherapy, immune-related adverse events, enterocolitis, hepatitis, myocarditis
Highlights
-
•
irAEs remain a significant management challenge.
-
•
The pathophysiology of irAEs is distinct to conventional autoimmune conditions.
-
•
Management must evolve to a more biological, targeted approach to improve outcomes.
Introduction
Over the past two decades, immunotherapy has become a cornerstone of treatment for multiple solid organ malignancies. Immune checkpoint inhibitors (ICIs) are monoclonal antibodies targeting anti-cytotoxic T-lymphocyte antigen-4 (CTLA4), anti-programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) and anti-lymphocyte activating-3 (LAG3). ICIs inhibit negative immune regulation pathways, inducing an augmented anticancer immune response. However, ICIs may cause collateral off-target immune-related adverse events (irAEs) affecting any organ system, which can limit ongoing ICI use for tumour control, impact quality of life and lead to significant morbidity and mortality. The risk of irAEs differs between ICI regimens, with approximate rates of grade ≥3 events of 7%-28% for single-agent anti-PD-1/anti-PD-L1 monotherapy1, 2, 3 versus 48%-59% for combination anti-PD-1/CTLA42, 3, 4 and 19% for combination PD-1/anti-LAG3.5
Multiple mechanisms have been proposed for irAE development, including unmasking of subclinical or latent autoimmunity, increasing T-cell recognition of antigens co-expressed on cancer and normal tissue and triggered immune dysregulation with overactivation of proinflammatory pathways and suppression of immune control pathways.6 Probability of irAE development is influenced by ICI agent, the organ-specific immune environment and primary tumour type (likely related to tumour neoantigens).7 irAEs are customarily graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0; however, as we gain experience with irAEs, more nuanced criteria are needed to guide management. Personalised treatment for irAEs is currently poorly defined, relying on untargeted immunosuppression initially with systemic corticosteroids to dampen immune activation. Several steroid-sparing agents have additionally been adopted, with efficacy extrapolated from the management of idiopathic autoimmune disorders. Importantly, these agents may have significant effects on immune signalling and may impact the antitumour effect of ICIs. Some patients with grade 3-4 irAEs still experience profound antitumour responses despite immunosuppression; however, the role of toxicity as a surrogate for overall survival benefit remains unclear.8 Some immunomodulatory agents may even positively influence antitumour response, with recent preclinical and early-phase evidence suggesting that concurrent anti-tumour necrosis factor-α (TNF-α) and ICI may enhance antitumour response through preventing anti-PD-1-induced tumour-infiltrating lymphocyte cell death.9, 10, 11 A validated clinical impact remains unclear, however. The primary goal of immunosuppressive therapy should be to mitigate ICI-related toxicity without reversing the antitumour effect of immunotherapy. This can only be achieved by better understanding of the pathophysiology and key inflammatory pathways involved to inform nuanced, targeted management paradigms.
Systemic corticosteroids have been the cornerstone of management of irAEs; however, as they induce apoptosis of proliferating T cells, they also have clinically relevant impacts on the therapeutic response to ICI.12 Several more targeted agents have shown efficacy in irAE management including anti-TNF-α monoclonal antibodies (infliximab), agents blocking T-cell DNA base production [mycophenolate mofetil (MMF)], interleukin (IL)-6 inhibitors (tocilizumab) and Janus kinase (JAK) inhibitors (tofacitinib). In severe refractory cases, untargeted agents that have profound effects on immune signalling may be employed, such as intravenous immunoglobulin and plasmapheresis to deplete antibodies, and anti-thymocyte globulin (ATG) leading to abrupt T-cell depletion. Evidence for many of these agents comes from case reports or small case series and may not report on the subsequent effect on cancer outcomes. Although escalation pathways are typically borrowed from treatment paradigms for conventional autoimmune conditions, higher doses of initial steroids and rapid escalation in steroid-refractory disease are often recommended13, 14, 15 as, unlike chronic autoimmune disease, >85% of non-endocrine irAEs may resolve with early treatment.3 A summary of recent immunomodulatory agents is provided in Table 1.
Table 1.
Immunomodulatory agents used in enterocolitis, hepatotoxicity and myocarditis
| Agent | Mechanism | Effect on antitumour response | Uses |
|---|---|---|---|
| Corticosteroids | Supressed T-cell activation, migration and differentiation | Likely negative effect early in ICI course or in high doses Inconclusive effect on survival outcomes11,16 |
Most irAEs |
|
Topically acting corticosteroids Budesonide Beclomethasone dipropionate |
Binds and activates glucocorticoid receptors, with limited systemic absorption due to high first-pass metabolism | Less negative impact than systemic corticosteroids | Microscopic colitis17,18 Hepatitis19 |
|
Ursodeoxycholic acid Supportive alongside steroids |
Various choleretic effects | No impact | Cholangitis20 |
| Mycophenolate mofetil | Inhibits proliferation of T and B lymphocytes | Inconclusive | Colitis Hepatitis Myocarditis21 |
|
Infliximab 5 mg/kg i.v., repeat 10 mg/kg i.v. if no response |
Anti-TNF-α Neutralisation of inflammatory macrophage-derived TNF-α |
Likely maintained9,10 | Colitis Hepatitis |
|
Vedolizumab 300 mg i.v. at weeks 0, 2 and 6, and then every 8 weeks |
Anti-α4β7 integrin Blocks interaction of α4β7 integrin with MadCAM-1, preventing T-cell gastrointestinal homing |
Likely no impact22 | Colitis22 |
|
Ustekinumab 40 mg, then 45 mg after 4 weeks and then every 12 weeks |
Anti-IL-12/23 Inhibits IL-12/23-mediated proinflammatory Th1 and Th17 expansion |
Inconclusive | Colitis23,24 Hepatitis25 |
|
Tofacitinib Ruxolitinib Alongside abatacept |
JAK-STAT pathway inhibition Reversal of CD8+ TRM cell activation and IFN-γ signalling |
Likely maintained 26,27 | Colitis26,28 Myocarditis29 |
|
Tocilizumab 4 mg/kg i.v., repeat dose within 48 h Onset: days |
Anti-IL-6 Inhibits IL-6-mediated proinflammatory T-cell expansion |
Possible positive effect30 | Hepatitis31 |
|
Abatacept Alongside ruxolitinib. 500-1000 mg i.v. weekly Onset: weeks |
CTLA4 agonist Binds B7 ligands preventing T-cell stimulation |
Inconclusive | Myocarditis29 |
| Faecal microbiota transplant | Favourable microbiota profile increases Treg cells, decreases proinflammatory T effector cells | Possible positive effect32 | Colitis33,34 |
CTLA4, cytotoxic T-lymphocyte antigen-4; i.v., intravenous; ICI, immune checkpoint inhibitor; IL, interleukin; JAK, Janus kinase; STAT, signal transducer and activator of transcription; Th1, T-helper 1; Th17, T-helper 17; TNF-α, tumour necrosis factor-α; Treg, T-regulatory; TRM, tissue resident memory T cells.
In this review, we focus on three key immune-related toxicities where recent clinical and translational work has provided nuanced insights into pathogenesis and treatment strategies: enterocolitis, hepatitis and cardiovascular toxicity including myocarditis. Enterocolitis and colitis are common toxicities occurring in 32%-37% of patients receiving doublet anti-PD-1 and anti-CTLA4 and 4%-6% of patients receiving anti-PD-1 monotherapy.35,36 Management of gastrointestinal irAEs contributes significantly to inpatient burden as the second most common irAE requiring hospitalisation (after pneumonitis), accounting for 17% of all irAE-related hospitalisations in one academic centre.37 Recent work has highlighted differences in pathogenesis compared to idiopathic inflammatory bowel disease (IBD), potentially informing new treatment options. Hepatic toxicity is common and recognition of clinical differences between hepatocellular and cholangitic patterns is helpful in guiding therapy.38 Myocarditis is much less common (<1%)39 but has an early onset and high relative mortality so timely recognition and prompt treatment are vital.
Enterocolitis
Gastrointestinal (GI) irAEs can involve any part of the GI tract, most commonly affecting the colon. Severe colitis occurs in ∼10%-20%, causing significant morbidity and leading to permanent discontinuation of ICI in just over half of patients impacted.40 Whilst mortality is estimated at 1%, it is the leading cause of death amongst all irAEs.41 Approximately 25% of patients with ICI colitis present with concomitant enteritis and 10% of patients may present with inflammation isolated to the upper GI tract (gastritis, gastroenteritis or enteritis),42 which can be more challenging to diagnose in practice.
Pathogenesis of enterocolitis
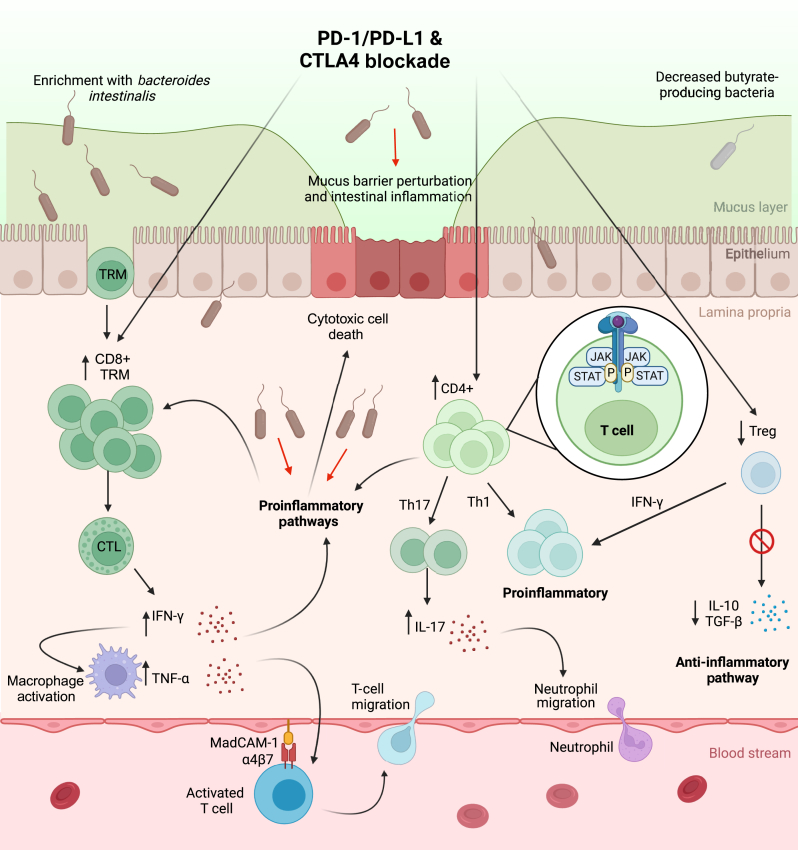
Recent work suggests that ICI colitis is a distinct entity from idiopathic IBD, with high levels of activated memory CD8+ T cells and mucosal-associated invariant T cells, not present in colitis-free patients treated with ICI or in patients with ulcerative colitis (UC).43 Activation and proliferation of mucosal CD8+ T cells stimulated by ICIs may induce recruitment of peripheral CD8+ and CD4+ T-cell populations, further driving ICI colitis.44 Tissue resident memory T (TRM) cells, usually located in colonic mucosa to respond rapidly to pathogens, are less pronounced in patients with IBD28,45 and aberrantly increased following ICI, with very high levels in patients with ICI colitis.43,44 The proportion of CD8+ TRM cell activation also positively correlates with clinical and endoscopic colitis severity.43 Notably, patients with ICI gastritis also have a high proportion of activated CD8+ TRM cells in gastric mucosa, reinforcing their role more broadly in ICI-driven GI inflammation. As a result of T-cell activation, proinflammatory signalling pathways are up-regulated in CD4+ and CD8+ T-cell clusters in ICI colitis.43,44,46 Patients with ICI colitis exhibit a lower proportion of T-regulatory (Treg) cells compared to patients with UC; however, current data are inconclusive as to whether Treg cells are consistently suppressed in ICI colitis.43,44 Nonetheless, the gene expression of Treg cells may change to a more T-helper 1 (Th1) proinflammatory profile in response to interferon-γ (IFN-γ), with subsequent loss of immune regulatory functions.44 In the presence of proinflammatory cytokines, CD4+ cells differentiate into mature, proinflammatory T-helper 17 (Th17) cells, responsible in part for chemokines mediating neutrophil recruitment.47
It is increasingly evident that the gut microbiome also plays a crucial role in irAEs. Bacteroides intestinalis has been found to be enriched in patients with CTCAE grade 3-4 ICI colitis irrespective of baseline microbiota diversity, up-regulating proinflammatory IL-1β in the mucosal environment in a murine model.48 However, conflicting data have not found an association between Bacteroides and colitis.49,50 The balance of butyrate-producing bacteria (including Faecalibacterium and Agathobacter genera) appears important and is associated with a lower incidence of irAEs, and lower incidence of colonic irAEs compared to non-colonic irAEs.17,18
The proposed mechanisms for ICI colitis are depicted in Figure 1. Collectively, these data show that ICI colitis is a distinct entity from IBD with different underlying pathogenesis and immune signalling dysregulation.
Figure 1.
Pathways involved in the pathogenesis of ICI colitis. ICI leads to widespread T-cell activation. Increased CD8+ TRM cells and activated CTLs result in release of proinflammatory cytokines including IFN-γ and, via activation of macrophages, TNF-α. Increased CD4+ Th17 cells secrete IL-17, a potent chemokine promoting neutrophil migration. CD4+ Treg cells convert to a proinflammatory Th1 profile under the influence of IFN-γ, losing immune regulatory properties. Proinflammatory pathways recruit further peripheral immune cells into the inflammatory microenvironment resulting in intestinal inflammation, cell death and disruption to colon integrity. The influence of the gut microbiome, particularly enrichment with Bacteroides intestinalis, contributes to the proinflammatory environment. Created with BioRender.com. CTLA4, cytotoxic T-lymphocyte antigen-4; CTLs, cytotoxic T-cell lymphocytes; ICI, immune checkpoint inhibitor; IFN-γ, interferon-γ; IL, interleukin; JAK, Janus kinase; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor-β; Th17, T-helper 17; TNF-α, tumour necrosis factor-α; Treg, T-regulatory; TRM, tissue resident memory T cells.
Approach to management
Despite newer evidence for differing pathophysiological mechanisms, management paradigms for ICI colitis are still largely borrowed from IBD, although steroids are used in higher doses and typically ICI colitis resolves without the need for ongoing immunosuppression unlike IBD. Guidelines for management are stratified based on CTCAE grading, assessing colitis severity based on diarrhoea frequency alone. This approach might underestimate severity, lacking consideration for endoscopic findings such as deep ulceration or colitis extent.51 Other scoring systems, such as the Mayo Endoscopic Scoring system, take into account both symptoms and endoscopic findings and are gaining popularity in clinical practice as a more accurate reflection of colitis severity.52 In two large retrospective studies, neither CTCAE diarrhoea grade >1 nor biomarkers predictive of clinical severity in IBD (C-reactive protein, albumin, haemoglobin) correlated with severity of ICI colitis or clinical course.22,35 Conversely, endoscopic and histologic evaluation was predictive of treatment outcome, with severity correlating with steroid duration and need for immunosuppression escalation.22,35 Endoscopy is additionally useful in adequately assessing histologic subtype of ICI colitis. ICI-related microscopic colitis has been recognised as a distinct entity in patients with minimal mucosal inflammation on endoscopy but lymphocyte-predominant infiltrate histologically and demonstrates responses to oral topically acting steroids (budesonide, beclomethasone dipropionate),23,24 questioning the need for systemic steroids in these patients. Early endoscopy may therefore be more helpful than clinical parameters to characterise disease severity and guide treatment strategy.
Currently, primary management of ICI colitis involves high-dose corticosteroids, addition of TNF-α blockade for steroid-resistant disease (occurring in ∼50%51) and temporary (if grade 2-3) or permanent (if grade ≥4) ICI discontinuation.13 In a case series of 10 patients, dose escalation of infliximab (10 mg/kg, after failure of at least one dose at 5 mg/kg) led to clinical response in 50% of patients,25 and may represent a viable option for refractory cases. Vedolizumab is often used as an alternative biologic in the initial treatment of steroid-refractory disease and mechanistically may have advantages as a gut-selective anti-α4β7 integrin agent with less off-target immunosuppressive effects. However, a slightly longer time to response (17.5 versus 13 days with infliximab, P = 0.012)26 may limit its utility in severe cases. In this study, overall survival was favourable with greater use of vedolizumab or infliximab rather than more steroid exposure. Novel integrin agents being explored for IBD (e.g. broader anti-β7 agent etrolizumab) may also have a role in ICI colitis as their efficacy becomes clearer. For the ∼20% of patients who are refractory to both steroids and initial biologic agents,51 the optimal third-line treatment is unclear.
Ustekinumab is a monoclonal antibody specific for the p40 subunit of IL-12 and IL-23, which is approved for use in psoriasis and IBD and has been successfully used in psoriasiform cutaneous irAE. Clinical evidence for its efficacy in ICI colitis has been described in case reports of patients with refractory colitis despite infliximab and vedolizumab.33,34 Recently, ustekinumab was used as a first-line biologic in a case of concurrent ICI colitis and ICI hepatitis to avoid the potential hepatotoxicity associated with infliximab, with successful resolution of both irAEs.25
Exploiting our understanding of the integral role of JAK and IFN-γ signalling in the colonic microenvironment, JAK1/3 inhibition with tofacitinib has shown promise in treatment-refractory ICI colitis, resulting in rapid resolution of recurrent ICI colitis and reversal of CD8+ TRM cell activation and IFN-γ signalling, whilst maintaining the antitumour response.46 Similarly, in a small case series of four patients, all patients achieved steroid-free remission of ICI colitis following tofacitinib, including one patient with disease refractory to steroids, infliximab, vedolizumab and ustekinumab.53 Of those who achieved cancer remission before initiation of tofacitinib (3 of 4), all remained cancer-free at follow-up (range 12-71 weeks), which is notable as JAK inhibition has potential to similarly deactivate intratumoural CD8+ TRM cells integral to the antitumour response.
Extracorporeal photophoresis induced remission in a case of refractory ICI colitis via expansion of natural killer cells and activation of an immune regulatory phenotype. This area requires more research but could represent a novel treatment option for the future.54
Faecal microbiota transplant (FMT) has a unique role in the management of ICI colitis without the need for systemic immunosuppression, building on emerging work on the microbiome. Case series have provided a foundation of evidence for FMT from healthy donors as salvage therapy for refractory cases of ICI colitis, with a clinical response rate of 73% in one series of 15 patients, although only 46% achieved endoscopic remission.55 Early interim results from a small series suggest that first-line FMT may be efficacious, providing symptom relief within days and allowed resumption of immunotherapy in four of seven patients.56 A prospective study is currently under way exploring the first-line use of FMT for ICI colitis, abrogating the need for systemic immunosuppression (NCT04038619). The wider role of FMT in regulating tumour immunity is an area of significant research,57 and further clinical evidence is required before FMT can be adopted into routine clinical practice or treatment algorithms.
Rechallenge and prophylaxis
Rechallenge may be considered in patients who have achieved clinical remission of their colitis depending on the severity of their initial ICI colitis. Despite clinical remission, persistent endoscopic inflammation is associated with an increased risk of developing recurrent colitis,58 and repeat colonoscopy may be warranted to stratify individual risk. Patients with ICI colitis may be less likely to have recurrent toxicity compared to other initial irAEs.59
Recurrence of colitis on rechallenge appears in part related to ICI agent used and sequence, with one series reporting colitis recurrence in 27% of patients switching from anti-CTLA4 to anti-PD-(L)1 monotherapies, whilst those switching from anti-PD-(L)1 to anti-CTLA4 experienced recurrence in 88%.60 Colitis may be particularly unlikely to reoccur when rechallenged with PD-1 monotherapy after combination ICI, occurring in only 6% (2 of 33 patients) in one series.59 At recurrence, colitis is typically not severe (grade ≤2), but the majority require systemic immunosuppression.60 ICI colitis may also occur de novo on rechallenge following a different initial irAE, newly occurring in 50% of patients (10 of 20) in a small retrospective series.61
There is a growing body of evidence for various agents for primary and secondary prophylaxis against GI irAEs. In the largest multicentre series to date, resumption of ICI with concurrent immunosuppression (n = 77; 33 infliximab, 44 vedolizumab) was associated with a reduced risk of recurrence of severe colitis or diarrhoea compared to controls (n = 61) rechallenged without immunosuppression (20.8% versus 34.4%, P = 0.036), with less severe endoscopic findings at recurrence and no difference in survival outcomes.62 Interestingly, results favoured the prophylaxis group despite a larger number of patients in the control group switching from anti-CTLA4 regimen to anti-PD-(L)1, typically associated with a lower risk of recurrence. There were lower rates of immunosuppression-related toxicity in the vedolizumab group (11.4% versus 18.9%) and numerically less recurrence in the vedolizumab group, but the study was not randomised or powered to draw conclusions between agents and warrants prospective investigation. Primary prophylaxis with budesonide has been explored in an early placebo-controlled study in advanced melanoma treated with ipilimumab, but did not find any significant difference in rates of diarrhoea.63
Hepatotoxicity
ICI-mediated hepatotoxicity generally presents as an asymptomatic acute transaminitis generally within 3 months of ICI initiation, with higher risk in anti-CTLA4 agents [odds ratio (OR) 5.01 versus 1.94 for anti-PD-1] and melanoma compared to other tumour types (OR 5.66 versus 2.71).64 Liver biopsy remains useful in patients not responding to steroid immunosuppression, primarily to rule out other causes of acute hepatitis [e.g. viral, autoimmune hepatitis (AIH), drug induced], and to establish the pattern of inflammation. Non-alcoholic fatty liver disease has emerged as a potential risk factor for the development of ICI hepatitis, even in the absence of being overweight according to body mass index.65
Pathogenesis of hepatotoxicity
ICI hepatitis represents a distinct pathology to conventional AIH on histopathological analysis. Interface hepatitis with piecemeal necrosis, plasma cell infiltration and rosette formation are characteristic of AIH,66 while ICI hepatitis is characterised by panlobular lymphocytic infiltrate (typically zone 3) and an absence of plasma cells and rosette formation.67 Hepatotoxicity from anti-CTLA4 agents may additionally feature granulomatous lesions associated with central vein endotheliitis and fibrin deposition.19
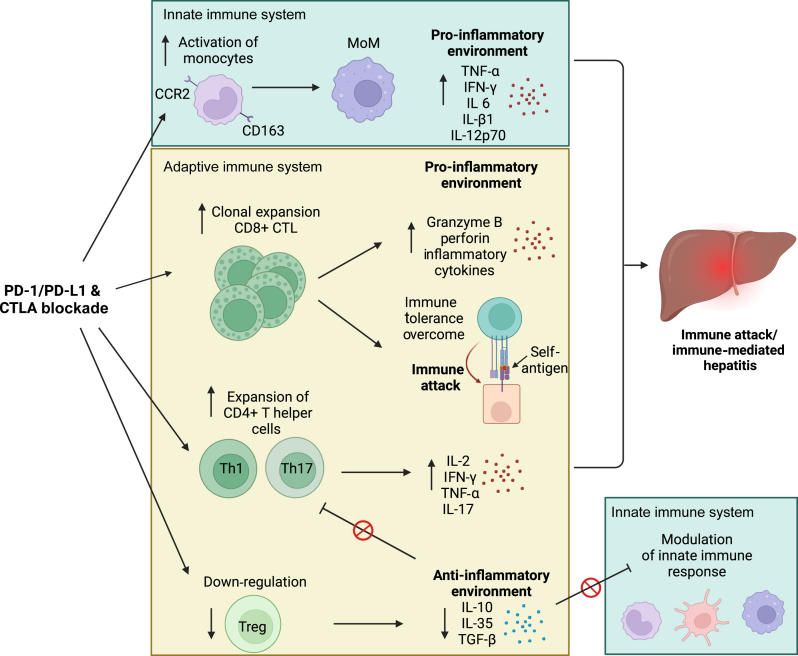
Proposed mechanisms for ICI hepatitis are shown in Figure 2. Due to the high antigen load of the portal circulation, the liver has a unique immune environment reliant on maintenance of immune tolerance, distinct from many other organs affected by irAE.68 Phenotypic and transcriptional profiling of peripheral immune subsets in patients with ICI hepatitis reveals increased CD8+ cytotoxic T lymphocytes (CTLs) in peripheral circulation and the liver inflammatory microenvironment, with resultant expression of proinflammatory effector molecules (intracellular granzyme B, perforin) proposed to result in hepatocyte damage.69 CD4+ T cells are also increased, with preferential clonal expansion of Th1 and Th17 CD4+ helper cells which secrete proinflammatory cytokines and stimulate the innate immune system.20 Loss of immune tolerance is compounded by suppression of Treg cells.60
Figure 2.
Pathways involved in the pathogenesis of ICI hepatitis. ICIs are proposed to cause clonal expansion of CD8+ CTLs and to a lesser extent expansion of Th1 and Th17 CD4+ T cells which may directly cause cytotoxic cell damage. Clonal expansion of CD8+ CTLs is proposed to overcome immune tolerance and indirectly cause cell death via loss of tolerance and erroneous cytotoxic targeting of self-antigens. The innate immune system may be key in ICI hepatitis via ICI activation of monocytes and an increased number of MoMs resulting in a proinflammatory environment resulting in hepatocyte apoptosis. Finally, suppression of Treg cells leads to loss of anti-inflammatory negative feedback. Created with BioRender.com. CTLA, cytotoxic T-lymphocyte antigen; CTLs, cytotoxic T lymphocytes; ICI, immune checkpoint inhibitor; MoM, monocyte-derived macrophages; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TGF-β, transforming growth factor-β; Th1, T-helper 1; Th17, T-helper 17; Treg, T-regulatory.
Cross-talk between the innate and adaptive immune systems in ICI hepatitis is suggested by co-localisation of CD68+CCR2+ (C-C chemokine receptor type 2+) macrophages and CD8+ CTLs in liver biopsies.69 Transcriptional profiling of patients with ICI hepatitis reveals an increase in circulating monocytes, with increased cell surface expression and soluble levels of CD163+ (representing an activated phenotype) and increased levels of tissue homing receptor CCR2 compared with healthy controls and hepatitis-free patients exposed to ICI.69 Monocyte-derived macrophages (MoMs) isolated from these patients in vitro expressed proinflammatory properties (secreting IFN-γ, IL-1β, IL-6, IL-12p70, TNF-α), suggesting that circulating monocytes may be recruited to the liver with increased differentiation into MoM and their proinflammatory effects may play a key role in ICI hepatitis.69
In contrast to a transaminitis, primary cholestatic derangement indicates dominant ICI cholangitis distinct to ICI hepatitis, which may be under-recognised in practice given its relative rarity.38 Distinct histologic subtypes of ICI cholangitis have emerged, with large-duct pathology defined by hypertrophy, dilation and fibrosis of the ductal wall and more significant elevation in alkaline phosphatase, while small duct disease exhibits a CD8+-predominate infiltrate in the small ducts leading to periductal fibrosis, retraction and bile duct loss that may not be evident on imaging.38 Recognising the pattern of liver toxicity—hepatocellular, cholestatic or mixed (cholangiohepatitis)—is an important step in predicting response to corticosteroids as ICI cholangiohepatitis is more refractory to steroids.31
Approach to management
Whilst active surveillance can be considered in a well patient with transient grade ≤2 derangement, corticosteroids remain an effective first-line management of persistent or severe (grade ≥3) ICI hepatitis.13 Evidence for the efficacy of budesonide in ICI hepatitis is growing, extrapolated from AIH, with a beneficial adverse effect profile compared to prednisolone.70 Approximately one-quarter of patients will have steroid-refractory disease71 unrelated to initial steroid dose, with similar hepatitis outcomes for patients receiving methylprednisolone 1 mg/kg/day compared to ≥1.5 mg/kg/day, although with reduced risk of steroid-related complications.21 ICI cholangitis is particularly refractory to corticosteroids (response rate 11.5%) but this may be improved when combined with ursodeoxycholic acid (response rate 28.6%),31 so should be considered early in the course for these patients. In most cases liver enzymes improve, but only a minority return to normal (<10%) and recovery is protracted with 90% of recovery occurring after 3 months.38
In the second line, infliximab is best avoided due to its own potential for drug-induced liver injury.72 Several management guidelines suggest MMF as a second-line agent for hepatotoxicity without robust evidence of superiority over other agents (e.g. azathioprine, with wider side-effect profile and need for blood test monitoring). There is no clear consensus regarding the timing of escalation—current European Society of Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) guidelines suggest addition of MMF after 48-72 h of no improvement,13,14 supported by a recent meta-analysis showing that time to return to normal was improved with the early addition of second-line immunosuppressants (median time to recovery 37.7 days versus 47.6 days for steroid monotherapy).73 In contrast, current Society for Immunotherapy of Cancer (SITC) guidelines advocate for initiation of second-line MMF at 10-14 days if no improvement.15 Third-line treatment is not defined, with case reports and series describing use of various agents such as tocilizumab, tacrolimus, cyclosporine, azathioprine and ATG. Cytokine-directed therapy with anti-IL-6 (tocilizumab) has shown promise in steroid-refractory ICI cholangiohepatitis, informed by an underlying immune signature on baseline biopsy high in proinflammatory cytokines (IFN-γ and induced chemokines including IL-6). In a case series of three patients with this biomarker profile, all achieved rapid clinical and biological remission after 1-2 doses.74
Rechallenge and prophylaxis
Evidence for rechallenge indicates a recurrence risk of 17%-35%59,75,76 with lowest rates reported after PD-(L)1 rechallenge following combination ICI59; however, comparison is limited given the heterogeneity between studies. In one report, 25% of recurrences were grade 4, and highest in those with documented bile duct injury,75 although another series found no association with previous pattern of liver injury.76 There are currently no prospective data to support prophylactic immunosuppression on rechallenging patients with ICI; however, when low-dose immunosuppression has been continued, it does not appear to modify the risk of hepatitis recurrence.75 Interestingly, in a prospective study, 75% of patients who experienced recurrence had an underlying autoimmune disorder or positive antinuclear antibody (titre ≥1/80) (versus 26.7% without, P = 0.037), which may serve as a biomarker for recurrence risk.71
Cardiovascular toxicity
Cardiac complications are relatively infrequent irAEs, although their low frequency is offset by high associated morbidity and mortality, with reported mortality rates up to 50%.77 Although myocarditis is the most well-known, it is increasingly recognised that cardiovascular complications of ICI are heterogeneous and include increased rates of acute coronary syndrome, pericardial disease and arrhythmias.29,78,79 The strongest established risk factor for cardiovascular complications is combination ICI.77 Prospective studies are increasingly recognising cardiovascular toxicity, likely under-identified in previous retrospective analyses. When studies incorporated regular troponin monitoring, higher rates of myocarditis have been noted—e.g. 1.7% in combination nivolumab and relatlimab and 0.6% with nivolumab monotherapy in RELATIVITY-0475 compared to no reported myocarditis in either combination ipilimumab and nivolumab, or nivolumab or ipilimumab groups in CheckMate-067 where routine cardiac monitoring was not employed.3 Pericardial disease accounts for 0.4% of adverse events attributed to ICI in pharmacovigilance studies, occurring fourfold higher in ICI-treated patients than in controls.29 Case reports of takotsubo cardiomyopathy secondary to ICI therapy have also been reported.80
Pathogenesis of cardiac toxicity
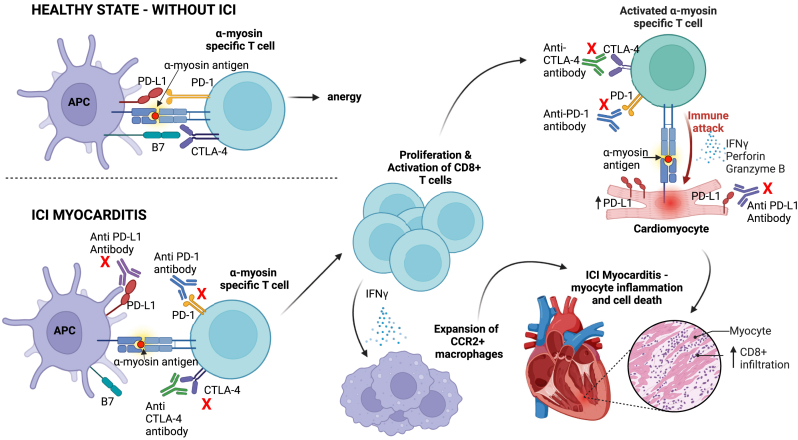
The underlying pathophysiology of ICI myocarditis is largely driven by clonal expansion of autoreactive cytotoxic CD8+ T cells specific for α-myosin in skeletal and cardiac muscle, leading to an extensive lymphohistiocytic infiltrate and myocyte necrosis81,82 (Figure 3). Cardiomyocyte PD-L1 expression is up-regulated in response to stress such as ischaemia or hypertrophy,83 and T-cell infiltration of the myocardium with detectable PD-1 and PD-L1 has been reported in cynomolgus monkey models following ICI treatment.84 Macrophages have also been implicated and an expansion of cardiac CCR2+ macrophages, which express an inflammatory phenotype, has been observed in both mice and humans with ICI myocarditis.85 The exact mechanism remains unclear, but it is proposed that CD8+ T cells producing IFN-γ lead to the differentiation of chemokine (C-X-C motif) ligand 9 (Cxcl9) and chemokine (C-X-C motif) ligand 10 (Cxcl10) CCR2+ macrophages from CCR2+ monocytes. Blocking IFN-γ signalling emerges as a prospective target for the treatment of ICI myocarditis in the future.
Figure 3.
Pathways involved in the pathogenesis of ICI myocarditis. In a healthy state, immune checkpoints (CTLA4, PD-1) maintain immune tolerance by preventing autoreactive T-cell activation in the presence of self-antigens (e.g. α-myosin) and maintain T-cell anergy. ICIs block immune checkpoints (red crosses), which may result in activation of autoreactive CD8+ T cells that evade central tolerance, clonally proliferate in the peripheral blood and directly attack α-myosin antigens on cardiomyocytes resulting in myocyte inflammation and cell death. Migration and expansion of CCR2+ macrophages and resulting proinflammatory cytokine release further contribute to myocyte inflammation. Created with BioRender.com. APC, antigen presenting cell; CTLA4, cytotoxic T-lymphocyte antigen-4; ICI, immune checkpoint inhibitor; IFN-γ, interferon-γ; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Relatively short time to symptom onset (median 31 days)77 and rapid improvement after corticosteroid administration further suggest that ICI myocarditis may not represent an off-target effect but rather a direct complication of shared immune-protection pathways between cancer and cardiomyocyte cells. This underpinning pathophysiology also explains concomitant myositis in the vast majority of patients (95% abnormal muscle biopsy), with a comparably high frequency of respiratory muscle involvement compared to other causes of myositis.86 The rare but often fatal overlap syndrome of myasthenia gravis, myositis and myocarditis is less well understood but is commonly seropositive for muscle-specific or myasthenia gravis-specific autoantibodies,14 and patients presenting with any one of this triad should be assessed for all three.15
Aside from myocarditis, ICI is also associated with fourfold increased risk of myocardial infarction suggesting that atherosclerotic complications may be common and frequently overlooked.78 Autopsy studies have demonstrated greater T-cell infiltrate within coronary arteries in ICI-treated patients compared to non-ICI patients,87 while increased inflammatory cytokines from activated T cells within atherosclerotic plaque may destabilise plaque, increasing infarction risk.
Approach to management
Assessment of pre-treatment risk and diagnosis of ICI myocarditis remain challenging due to heterogeneity in presentation and absence of accurate and specific tests. Less than half of patients have reduced left ventricular ejection fraction (LVEF <50%) on presentation,77 limiting the value of routine echocardiography. Advanced imaging techniques for tissue characterisation such as cardiac magnetic resonance imaging (MRI) are impeded by limited access,88 may be non-diagnostic at presentation or have non-specific abnormalities (present in one-third of patients with cancer even before ICI initiation).16,27 Serum biomarkers such as troponin-I are sensitive for myocarditis but lack specificity.
Up to half of patients initially suspected of ICI myocarditis will have an alternative case found, reinforcing the importance of expedited and thorough diagnostic work-up; however, this must be balanced with the need for early immunosuppressive therapy.30 Initial work-up ideally includes serial biomarkers including troponin, brain natriuretic peptide and creatine kinase, serial electrocardiograms, at least 24 h of telemetry, echocardiography (ideally three-dimensional LVEF and global longitudinal strain measurement) and preferably cardiac MRI. Invasive or computed tomography (CT) coronary angiography, CT pulmonary arteriography or endomyocardial biopsy should also be pursued in patients with risk factors, a consistent clinical picture for an alternative diagnosis, or where the diagnosis is unclear.
Management guidelines for ICI myocarditis are largely based on myocarditis of different aetiology.89 Once ICI myocarditis appears likely, high-dose intravenous methylprednisolone (500-1000 mg daily) should be commenced. Early steroid initiation is important, with improved outcomes in patients treated within 24 h versus >72 h after symptom onset.90 Despite initial rapid clinical improvement, up to 67% are steroid refractory91 and guidance on efficacious second-line agents is limited.14 Despite recommendation in some guidelines,14 evidence for infliximab in ICI myocarditis is largely taken from case reports and there is concern around the association between TNF-α agents and cardiovascular events. Alterative second-line agents include MMF, tocilizumab or ATG.13, 14, 15 A recent and promising strategy involves combination JAK inhibitor (ruxolitinib) to rapidly suppress T-cell cytokine sensing and proliferation, and anti-CD80/86 CTLA4-immunoglobulin (abatacept) with a delayed yet more durable suppression of T-cell expansion.86 Within this small study of 40 patients, only 1 (3.4%) died from myocarditis (versus historic rates of 60%). Importantly, active surveillance for respiratory muscle involvement was also employed which likely contributed to improved outcomes. Abatacept is now the subject of a prospective phase III, placebo-controlled study (NCT05335928) where it will be compared to placebo in patients with ICI myocarditis treated with methylprednisolone to assess whether major adverse cardiac events are reduced. This is a step towards interrogating management strategies in prospective large multicentre randomised controlled trials. Further research is required to more accurately identify high-risk individuals and inform effective cardiac monitoring strategies.88
Rechallenge and prophylaxis
ICI rechallenge following confirmed ICI myocarditis is a vexing clinical issue with little in way of prospective studies for guidance, and should ideally take place in a multidisciplinary context89 personalised to each patient depending on severity, resolution and availability of alternative anticancer strategies. For patients initially treated with combination ICI, rechallenge with monotherapy may be reasonable in selected cases alongside an aggressive cardiac monitoring regimen.92
Conclusion
The incidence and management of irAEs remains a significant challenge when treating patients with ICIs and will become increasingly relevant as the scope of indications for ICIs broadens. Several mechanisms underpin development of irAEs, from shared antigenicity in myocarditis, cytokine-mediated damage in colitis and dysregulation of immune homeostasis and cytotoxic T-cell activation and infiltration in hepatitis. As translational evidence is gathered across multiple irAEs, it is becoming clear that the drivers of ICI-related toxicity differ from conventional autoimmune conditions and the approach to management needs to move beyond historic treatment paradigms. The initial approach with high-dose corticosteroids and rapid escalation of immunosuppression in the absence of response to steroids compared to conventional autoimmune conditions is warranted for irAEs as we have the opportunity to resolve toxicity, unlike in chronic autoimmune illness. A key future direction in the management of irAEs relies upon personalised profiling of the multiple potential tumour and host variables interacting with the host immune response in the context of ICI treatment. Development of preclinical models, whilst challenging in this space, will allow for greater mechanistic insights and more nuanced therapeutic strategies to be tested.93 Further work to support immunomodulation for toxicity without abrogating the antitumour response of ICI is crucial to improving patient outcomes and rates of cure.
Acknowledgments
Funding
None declared.
Disclosure
LS reports advisory honoraria and speakers’ fees from Bristol Myers Squibb and Ipsen. All other authors have declared no conflicts of interest.
References
- 1.Robert C., Hwu W.J., Hamid O., et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur J Cancer. 2021;144:182–191. doi: 10.1016/j.ejca.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–137. doi: 10.1200/JCO.21.02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albiges L., Tannir N.M., Burotto M., et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6) doi: 10.1136/esmoopen-2020-001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawbi H.A., Schadendorf D., Lipson E.J., et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y., Yu S., Qiao H. Understanding the functional inflammatory factors involved in therapeutic response to immune checkpoint inhibitors for pan-cancer. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.990445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amoroso V., Gallo F., Alberti A., et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors’ efficacy: a systematic review and meta-analysis of randomized studies. ESMO Open. 2023;8(2) doi: 10.1016/j.esmoop.2023.100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer N., Lusque A., Virazels M., et al. 846P Triple combination of ipilimumab + nivolumab + anti-TNF in treatment naive melanoma patients: final analysis of TICIMEL, a phase Ib prospective clinical trial. Ann Oncol. 2022;33:S936–S937. [Google Scholar]
- 10.Bertrand F., Montfort A., Marcheteau E., et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. 2017;8:2256. doi: 10.1038/s41467-017-02358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Ruiz E., Minute L., Otano I., et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569(7756):428–432. doi: 10.1038/s41586-019-1162-y. [DOI] [PubMed] [Google Scholar]
- 12.Bai X., Hu J., Warner A.B., et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin Cancer Res. 2021;27(21):5993–6000. doi: 10.1158/1078-0432.CCR-21-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haanen J., Obeid M., Spain L., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(12):1217–1238. doi: 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer J.R., Abu-Sbeih H., Ascierto P.A., et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann L.H., Cautela J., Palaskas N., et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor–associated myocarditis: a narrative review. JAMA Cardiol. 2021;6(11):1329–1337. doi: 10.1001/jamacardio.2021.2241. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Tang H., Zhou Q., et al. Gut microbiota composition in patients with advanced malignancies experiencing immune-related adverse events. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1109281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson R.C., Shanahan E.R., Batten M., et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat Med. 2022;28(11):2344–2352. doi: 10.1038/s41591-022-01965-2. [DOI] [PubMed] [Google Scholar]
- 19.De Martin E., Michot J.M., Papouin B., et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Yang H., Yao Z., Zhou X., Zhang W., Zhang X., Zhang F. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213 doi: 10.1016/j.clim.2020.108377. [DOI] [PubMed] [Google Scholar]
- 21.Li M., Wong D., Vogel A.S., et al. Effect of corticosteroid dosing on outcomes in high-grade immune checkpoint inhibitor hepatitis. Hepatology. 2022;75(3):531–540. doi: 10.1002/hep.32215. [DOI] [PubMed] [Google Scholar]
- 22.Geukes Foppen M.H., Rozeman E.A., Van Wilpe S., et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1) doi: 10.1136/esmoopen-2017-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes M.S., Molina G.E., Chen S.T., et al. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):292. doi: 10.1186/s40425-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander J.L., Ibraheim H., Richards C., et al. Oral beclomethasone dipropionate is an effective treatment for immune checkpoint inhibitor induced colitis. J Immunother Cancer. 2022;10:5490. doi: 10.1136/jitc-2022-005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris J.P., Postow M.A., Faleck D.M. Efficacy of infliximab dose escalation in patients with refractory immunotherapy-related colitis: a case series. Oncologist. 2022;27(4):e350–e352. doi: 10.1093/oncolo/oyac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou F., Faleck D., Thomas A., et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9(11) doi: 10.1136/jitc-2021-003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faron A., Isaak A., Mesropyan N., et al. Cardiac MRI depicts immune checkpoint inhibitor-induced myocarditis: a prospective study. Radiology. 2021;301(3):602–609. doi: 10.1148/radiol.2021210814. [DOI] [PubMed] [Google Scholar]
- 28.Martin J.C., Chang C., Boschetti G., et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178(6):1493–1508.e20. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong J., Drobni Z.D., Zafar A., et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ederhy S., Devos P., Pinna B., et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging for the diagnosis of immune checkpoint inhibitor-associated myocarditis. Arch Cardiovasc Dis. 2022;115(2):114–116. doi: 10.1016/j.acvd.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Onoyama T., Takeda Y., Yamashita T., et al. Programmed cell death-1 inhibitor-related sclerosing cholangitis: a systematic review. World J Gastroenterol. 2020;26(3):353. doi: 10.3748/wjg.v26.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández-Gordón Sánchez F.M., Gómez-Domínguez E., Paredes Ruiz D., et al. Ustekinumab for corticodependent immune-mediated colitis by pembrolizumab, an alternative for patients with concomitant liver injury. Rev Esp Enferm Dig. 2022;114(6):356–357. doi: 10.17235/reed.2022.8618/2022. [DOI] [PubMed] [Google Scholar]
- 33.Thomas A.S., Ma W., Wang Y. Ustekinumab for refractory colitis associated with immune checkpoint inhibitors. N Engl J Med. 2021;384(6):581–583. doi: 10.1056/NEJMc2031717. [DOI] [PubMed] [Google Scholar]
- 34.Thomas A.S., Lee S.E., Shatila M., et al. IL12/23 blockade for refractory immune-mediated colitis: 2-center experience. Am J Gastroenterol. 2023;118(9):1679–1683. doi: 10.14309/ajg.0000000000002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung V.T.F., Gupta T., Olsson-Brown A., et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br J Cancer. 2020;123:207–215. doi: 10.1038/s41416-020-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 37.Balaji A., Zhang J., Wills B., et al. Immune-related adverse events requiring hospitalization: spectrum of toxicity, treatment, and outcomes. J Oncol Pract. 2019;15(9):e825. doi: 10.1200/JOP.18.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pi B., Wang J., Tong Y., Yang Q., Lv F., Yu Y. Immune-related cholangitis induced by immune checkpoint inhibitors: a systematic review of clinical features and management. Eur J Gastroenterol Hepatol. 2021;33(suppl 1):e858. doi: 10.1097/MEG.0000000000002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain P., Gutierrez Bugarin J., Guha A., et al. Cardiovascular adverse events are associated with usage of immune checkpoint inhibitors in real-world clinical data across the United States. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han C.Y., Fitzgerald C., Lee M., et al. Association between toxic effects and survival in patients with cancer and autoimmune disease treated with checkpoint inhibitor immunotherapy. JAMA Oncol. 2022;8(9):1352–1354. doi: 10.1001/jamaoncol.2022.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes M.S., Zheng H., Zubiri L., et al. Colitis after checkpoint blockade: a retrospective cohort study of melanoma patients requiring admission for symptom control. Cancer Med. 2019;8(11):4986–4999. doi: 10.1002/cam4.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasson S.C., Zaunders J.J., Nahar K., et al. Mucosal-associated invariant T (MAIT) cells are activated in the gastrointestinal tissue of patients with combination ipilimumab and nivolumab therapy-related colitis in a pathology distinct from ulcerative colitis. Clin Exp Immunol. 2020;202(3):335–352. doi: 10.1111/cei.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luoma A.M., Suo S., Williams H.L., et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell. 2020;182(3):655–671.e22. doi: 10.1016/j.cell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smillie C.S., Biton M., Ordovas-Montanes J., et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178(3):714–730.e22. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasson S.C., Slevin S.M., Cheung V.T.F., et al. Interferon-gamma-producing CD8+ tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. Gastroenterology. 2021;161(4):1229–1244.e9. doi: 10.1053/j.gastro.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bamias G., Delladetsima I., Perdiki M., et al. Immunological characteristics of colitis associated with anti-CTLA-4 antibody therapy. Cancer Invest. 2017;35(7):443–455. doi: 10.1080/07357907.2017.1324032. [DOI] [PubMed] [Google Scholar]
- 48.Andrews M.C., Duong C.P.M., Gopalakrishnan V., et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432–1441. doi: 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubin K., Callahan M.K., Ren B., et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7(1) doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaput N., Lepage P., Coutzac C., et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 51.Powell N., Ibraheim H., Raine T., et al. British Society of Gastroenterology endorsed guidance for the management of immune checkpoint inhibitor-induced enterocolitis. Lancet Gastroenterol Hepatol. 2020;5(7):679–697. doi: 10.1016/S2468-1253(20)30014-5. [DOI] [PubMed] [Google Scholar]
- 52.Dougan M., Wang Y., Rubio-Tapia A., Lim J.K. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology. 2021;160(4):1384–1393. doi: 10.1053/j.gastro.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 53.Bishu S., Melia J., Sharfman W., Lao C.D., Fecher L.A., Higgins P.D.R. Efficacy and outcome of tofacitinib in immune checkpoint inhibitor colitis. Gastroenterology. 2021;160(3):932–934.e3. doi: 10.1053/j.gastro.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apostolova P., Unger S., von Bubnoff D., Meiss F., Becher B., Zeiser R. Extracorporeal photopheresis for colitis induced by checkpoint-inhibitor therapy. N Engl J Med. 2020;382(3):294–296. doi: 10.1056/NEJMc1912274. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Ma W., Abu-Sbeih H., Jiang Z.D., DuPont H.L. Fecal microbiota transplantation (FMT) for immune checkpoint inhibitor induced–colitis (IMC) refractory to immunosuppressive therapy. 2020;38(suppl 15):3067. [Google Scholar]
- 56.Wang Y., Varatharajalu K., Shatila M., Campbell M.T., Msaouel P., Kovitz C.A. First-line treatment of fecal microbiota transplantation for immune-mediated colitis. 2023;41(suppl 16) 2510-2510. [Google Scholar]
- 57.Xu H., Cao C., Ren Y., et al. Antitumor effects of fecal microbiota transplantation: implications for microbiome modulation in cancer treatment. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.949490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abu-Sbeih H., Ali F.S., Wang X., et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7(1):93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollack M.H., Betof A., Dearden H., et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi: 10.1093/annonc/mdx642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Sbeih H., Ali F.S., Naqash A.R., et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol. 2019;37(30):2738–2745. doi: 10.1200/JCO.19.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolladille C., Ederhy S., Sassier M., et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badran Y.R., Zou F., Durbin S.M., et al. Concurrent immune checkpoint inhibition and selective immunosuppressive therapy in patients with immune-related enterocolitis. J Immunother Cancer. 2023;11(6) doi: 10.1136/jitc-2023-007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber J., Thompson J.A., Hamid O., et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Lie P., Guo M., He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Cancer. 2017;141(5):1018–1028. doi: 10.1002/ijc.30678. [DOI] [PubMed] [Google Scholar]
- 65.Sawada K., Hayashi H., Nakajima S., Hasebe T., Fujiya M., Okumura T. Non-alcoholic fatty liver disease is a potential risk factor for liver injury caused by immune checkpoint inhibitor. J Gastroenterol Hepatol. 2020;35(6):1042–1048. doi: 10.1111/jgh.14889. [DOI] [PubMed] [Google Scholar]
- 66.Ziemer M., Koukoulioti E., Beyer S., Simon J.C., Berg T. Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol. 2017;66(3):657–659. doi: 10.1016/j.jhep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Zen Y., Yeh M.M. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Path. 2018;31(6):965–973. doi: 10.1038/s41379-018-0013-y. [DOI] [PubMed] [Google Scholar]
- 68.Shojaie L., Ali M., Iorga A., Dara L. Mechanisms of immune checkpoint inhibitor-mediated liver injury. Acta Pharm Sin B. 2021;11(12):3727. doi: 10.1016/j.apsb.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gudd C.L.C., Au L., Triantafyllou E., et al. Activation and transcriptional profile of monocytes and CD8+ T cells are altered in checkpoint inhibitor-related hepatitis. J Hepatol. 2021;75(1):177–189. doi: 10.1016/j.jhep.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Eleftheriotis G., Skopelitis E. Immune-checkpoint inhibitor-associated grade 3 hepatotoxicity managed with enteric-coated budesonide monotherapy: a case report. Medicine. 2022;101(31) doi: 10.1097/MD.0000000000029473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xing H., Wang Y., Qu B., et al. The current status of steroid-refractory immune-checkpoint-inhibitor-related hepatotoxicity. Transl Oncol. 2023;28 doi: 10.1016/j.tranon.2023.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Infliximab. LiverTox: clinical and research information on drug-induced liver injury. 2017. Available at: https://www.ncbi.nlm.nih.gov/books/NBK548203/. Accessed December 12, 2023.
- 73.Chen K., He J., Xu J., Chen J. Effectiveness of immunosuppressant use for the treatment of immune checkpoint inhibitor-induced liver injury: a systematic review and meta-analysis. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1088741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moi L., Bouchaab H., Mederos N., et al. Personalized cytokine-directed therapy with tocilizumab for refractory immune checkpoint inhibitor–related cholangiohepatitis. J Thorac Oncol. 2021;16(2):318–326. doi: 10.1016/j.jtho.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Hountondji L., De Matos C.F., Lebossé F., et al. Clinical pattern of checkpoint inhibitor-induced liver injury in a multicentre cohort. JHEP Rep. 2023;5(6) doi: 10.1016/j.jhepr.2023.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riveiro-Barciela M., Barreira-Díaz A., Callejo-Pérez A., et al. Retreatment with immune checkpoint inhibitors after a severe immune-related hepatitis: results from a prospective multicenter study. Clin Gastroenterol Hepatol. 2023;21(3):732–740. doi: 10.1016/j.cgh.2022.03.050. [DOI] [PubMed] [Google Scholar]
- 77.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142(24):2299. doi: 10.1161/CIRCULATIONAHA.120.049981. 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong J., Drobni Z.D., Alvi R.M., et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer. 2021;158:99–110. doi: 10.1016/j.ejca.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serzan M., Rapisuwon S., Krishnan J., Chang I.C., Barac A. Takotsubo cardiomyopathy associated with checkpoint inhibitor therapy: endomyocardial biopsy provides pathological insights to dual diseases. JACC CardioOncol. 2021;3(2):330–334. doi: 10.1016/j.jaccao.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Axelrod M.L., Meijers W.C., Screever E.M., et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. 2022;611:818–826. doi: 10.1038/s41586-022-05432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baban B., Liu J.Y., Qin X., Weintraub N.L., Mozaffari M.S. Upregulation of programmed death-1 and its ligand in cardiac injury models: interaction with GADD153. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji C., Roy M.D., Golas J., et al. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin Cancer Res. 2019;25(15):4735–4748. doi: 10.1158/1078-0432.CCR-18-4083. [DOI] [PubMed] [Google Scholar]
- 85.Ma P., Liu J., Qin J., et al. Expansion of pathogenic cardiac macrophages in immune checkpoint inhibitor myocarditis. Circulation. 2024;149:48–66. doi: 10.1161/CIRCULATIONAHA.122.062551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salem J.E., Bretagne M., Abbar B., et al. Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discov. 2023;13(5):1100–1115. doi: 10.1158/2159-8290.CD-22-1180. [DOI] [PubMed] [Google Scholar]
- 87.Newman J.L., Stone J.R. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc Pathol. 2019;43 doi: 10.1016/j.carpath.2019.107148. [DOI] [PubMed] [Google Scholar]
- 88.Moslehi J., Salem J.E. Immune checkpoint inhibitor myocarditis treatment strategies and future directions. JACC CardioOncol. 2022;4(5):704–707. doi: 10.1016/j.jaccao.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lyon A.R., López-Fernánde T., Couch L.S., et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L., Zlotoff D.A., Awadalla M., et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141(24):2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang C., Lin J., Wang Y., et al. Case series of steroid-resistant immune checkpoint inhibitor associated myocarditis: a comparative analysis of corticosteroid and tofacitinib treatment. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.770631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coustal C., Vanoverschelde J., Quantin X., et al. Prognosis of immune checkpoint inhibitors-induced myocarditis: a case series. J Immunother Cancer. 2023;11(5) doi: 10.1136/jitc-2022-004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bayless N.L., Bluestone J.A., Bucktrout S., et al. Development of preclinical and clinical models for immune-related adverse events following checkpoint immunotherapy: a perspective from SITC and AACR. J Immunother Cancer. 2021;9:2627. doi: 10.1136/jitc-2021-002627. [DOI] [PMC free article] [PubMed] [Google Scholar]