Abstract
Obstructive sleep apnoea (OSA) can occur in both rapid eye movement (REM) and non-REM sleep or be limited to REM sleep, when the upper airway is most prone to collapse due to REM sleep atonia. Respiratory events are usually longer and more desaturating in REM than in NREM sleep. The prevalence of REM OSA is higher in women than in men and REM OSA usually occurs in the context of mild–moderate OSA based on the apnoea–hypopnoea index calculated for the entire sleep study. Studies have highlighted some detrimental consequences of REM OSA; for example, its frequent association with systemic hypertension and a degree of excessive daytime sleepiness similar to that found in nonsleep-stage-dependent OSA. Moreover, REM OSA could increase cardiometabolic risk. Continuous positive airway pressure (CPAP) treatment aimed at preventing REM OSA should be longer than the 4 h usually considered as good compliance, since REM sleep occurs mostly during the second half of the night. Unfortunately, patients with REM OSA show poor adherence to CPAP. Alternative non-CPAP treatments might be a good choice for REM OSA, but data are lacking. This review summarises the available data on REM OSA and critically examines the weaknesses and strengths of existing literature.
Shareable abstract
Obstructive sleep apnoea (OSA) occurring only in REM sleep (REM OSA) is often identified, especially in women with mild–moderate OSA. REM OSA appears consistently linked with hypertension but its overall impact on health is still poorly defined. https://bit.ly/3RFxIuB
Introduction
Obstructive sleep apnoea (OSA) is a highly prevalent medical condition, with an estimated global prevalence of almost 1 billion people, and rates exceeding 50% in some countries [1]. The pathogenesis of OSA is complex and its clinical manifestations are heterogeneous. Many factors affect its expression, including age, sex, ethnicity, bodily characteristics, comorbidities and sleep posture, as well as sleep stage, all of which collectively contribute to defining the endotypic and phenotypic features of OSA.
OSA may deteriorate during rapid eye movement (REM) sleep [2, 3]. Often, the number and duration of apnoeas and hypopnoeas increase during REM sleep compared with non-REM (NREM) sleep. In some patients, respiratory events occur primarily during REM sleep, a condition called REM sleep-related OSA (REM OSA). These patients might be symptomatic, yet they may not be diagnosed with OSA because their overall apnoea–hypopnoea index (AHI) falls within the normal range [4]. At present, there is no generally accepted definition of REM OSA and several definitions have been proposed [5–7]. Some authors make a distinction between REM-predominant (REMp) and REM-isolated (REMi) OSA. Yet other investigators focus on NREM versus REM OSA to assess the differential effect of both conditions [8, 9]. A recent comprehensive review of REM OSA highlighted the differences between NREM and REM sleep, and the features of respiratory events according to sleep stages [10]. The scope of this review is to examine the evidence underpinning the assertion that REM OSA may constitute a different phenotype in the heterogeneous constellation of OSA.
Physiology
NREM and REM stages alternate cyclically during nocturnal sleep. REM sleep constitutes on average 20–25% of total sleep time and is more prominent towards the later hours of the sleep period. It is a distinctive sleep state, characterised by low-amplitude, mixed-frequency electroencephalographic activity, general muscle atonia and episodic sequences of REMs [11], which define phasic REM sleep, as opposed to tonic REM sleep. Fluctuations in heart rate, blood pressure (BP) and sympathetic outflow appear during REM sleep [12]. Respiration is also unstable, with variations in respiratory frequency and tidal volume. Episodic reduction of ventilation often occurs in association with bursts of REMs and is related to the inhibition of the respiratory muscles, including the diaphragm [13, 14]. In that context, short central apnoeas and hypopnoeas are a common sign of the ventilatory instability of normal REM sleep.
Sleep is hallmarked by reduced ventilatory responses to hypoxaemia and hypercapnia, which are present during NREM sleep and become most pronounced during REM sleep, where reductions to less than a third of the wakefulness state responses may be observed [15, 16]. In OSA, this reduced ventilatory drive may explain why respiratory events last longer in REM sleep, thus leading to greater dips in oxygen saturation [2, 17]. Moreover, lung volumes are decreased in REM sleep, even in OSA patients treated with continuous positive airway pressure (CPAP) [18, 19]. The resultant reduction in pulmonary oxygen stores makes individuals more susceptible to rapid oxygen desaturation induced by respiratory events [20]. Knowing that hypoxia has potent pressor effects in OSA [21] and that hypoxia often exacerbates during REM sleep, it becomes clear why greater fluctuations in heart rate and BP can be observed in REM sleep as compared with NREM sleep.
The duration of obstructive apnoeas and hypopnoeas is determined by the chemical ventilatory drive and the arousal threshold [22]. The latter is linked to an incremental ventilatory effort that triggers a cortical arousal when a critical level of respiratory muscle force has been reached [23]. Ventilatory effort can be assessed by measuring oesophageal pressure, gauging intrathoracic pressure [24]. Using oesophageal pressure measurements, Gleeson et al. [23] demonstrated that the stimulus to arousal from sleep in normal subjects is independent of the source of the rising drive to breathe, be it hypoxia, hypercapnia or resistive loading. While large interindividual differences exist, the intraindividual arousal threshold proves fairly stable. In 116 consecutive OSA patients, indices of respiratory effort were significantly lower in REM than in NREM sleep [25], as shown by the values of peak negative intrathoracic pressure (39.6±1.9 mbar in REM sleep versus 50.9±2.5 mbar in NREM sleep). Less respiratory effort might allow apnoeas to last longer before reaching the arousal threshold, thus explaining the extended duration of respiratory events in REM sleep.
The loop gain of the ventilatory control system is a nonanatomical factor in the pathogenesis of OSA [22]. It has been shown that worse ventilatory control stability due to increased loop gain is present in NREM-predominant OSA. In contrast, patients with REMp OSA are more prone to passive upper airway (UA) collapsibility as compared with the NREM OSA phenotype [26].
The general suppression of the skeletal muscle tone during REM sleep also affects the dilating muscles of the UA. In particular, both phasic and tonic activity of the genioglossal muscle are decreased, predisposing to increased UA collapsibility [26–29]. Just like in healthy individuals of both genders, OSA patients under CPAP treatment showed a progressive decrease in the activity of the genioglossal muscle, transitioning from stable NREM to tonic REM and finally to phasic REM sleep [30]. From this observation, it was postulated that a generalised reduction in genioglossal activity during REM sleep predisposes to pharyngeal collapse, especially in those individuals who are critically dependent on UA dilator muscle activity for maintaining UA patency [30]. Recently, pharyngeal collapsibility, characteristic of REM sleep, was shown to be largely explained by ventilatory drive withdrawal rather than by particular decrements in muscle activity or responsiveness [31]. The final common pathway of these mechanisms is an increased propensity for complete REM sleep-related UA collapse of longer duration, as compared with NREM sleep.
Epidemiology
A high degree of heterogeneity exists in the definitions of REM OSA in epidemiological studies, as listed in table 1 and reported in tables 2 and 3.
TABLE 1.
Definitions of rapid eye movement (REM) obstructive sleep apnoea (OSA) in epidemiological studies
| Reference(s) | Definition of OSAREM |
| [32] | Quartiles of AHIREM, AHINREM ≥ or <8 events·h−1 |
| [33 ] | AHIREM ≥5 events·h−1, AHI <15 events·h−1 |
| [40] | No OSAREM: AHIREM <10 events·h−1; mild OSAREM: AHIREM 10–19 events·h−1; moderate OSAREM: AHIREM 20–29 events·h−1; severe OSAREM: AHIREM >30 events·h−1 |
| [35] | REM sleep ≥30 min; AHIREM categories (>5, 5.0–9.9, 10–19.9 and ≥20 events·h−1) |
| [41] | AHINREM <5 events·h−1; AHIREM: <5 (normal), 5.0–14.9 (mild), 15.0–29.9 (moderate) and ≥30.0 events·h−1 (severe) |
| [36, 39] | REM sleep ≥30 min; severe OSAREM: AHIREM >30 events·h−1 |
| [36] | REM sleep ≥30 min; severe OSAREM: AHIREM >30 events·h−1; total AHI <15 events·h−1 |
| [36] | REM sleep ≥30 min; severe OSAREM: AHIREM >30 events·h−1; total AHI <15 events·h−1 and AHINREM <5 events·h−1 |
| [38] | AHIREM >5 events·h−1; REM sleep ≥15 min |
| [43, 44] | Overall AHI between 10 and 25 events·h−1, AHIREM/AHINREM >2 and AHINREM <10 events·h−1 |
| [45] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM >2, REM sleep ≥15% of TST |
| [55] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, REM sleep ≥10 min |
| [46, 51, 56] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, REM sleep ≥10 min, AHINREM <15 events·h−1 |
| [53, 57, 42, 58, 48] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, REM sleep >10.5 min, AHINREM <8 events·h−1 |
| [57, 42, 54, 58, 48, 52] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2 |
| Predominant OSAREM [57, 42, 58, 47, 48, 50, 52] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, AHINREM <15 events·h−1 |
| Isolated OSAREM [34, 37, 54, 49] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, AHINREM <5 events·h−1, AHIREM >5 events·h−1, REM sleep ≥30 min |
| [49, 52] | Overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, AHINREM <15 events·h−1, REM sleep ≥30 min |
AHI: apnoea–hypopnoea index; NREM: non-REM; TST: total sleep time.
TABLE 2.
Studies on rapid eye movement (REM) obstructive sleep apnoea (OSA) in the general population
| First author [ref.], year | Study type; sample | Diagnostic criteria | Outcomes | Results |
| Chami [32], 2010 | Cross-sectional; 5649 subjects (mean age 62.5 years, 52.6% women), SHHS | Quartiles of AHIREM in patients with AHINREM ≥ or <8 events·h−1 | ESS score, QoL and subjective sleep disruption | Prevalence of OSAREM: 74% in the analysed samples. In fully adjusted models, ESS scores associated with increasing AHINREM but not with AHIREM. Similar results for mental and physical QoL and report of insomnia. |
| Khan [33], 2013 | Cross-sectional; 2765 men (age ≥65 years), outcomes of MrOS Sleep study | REM-predominant OSA: AHI <15 events·h−1, with AHIREM ≥5; analysis stratified by AHIREM <5, 5– <15, 15– <30 and ≥30 events·h−1 | ESS, FOSQ, PSQI, SF-12, GDS and self-perceived health status | Prevalence of OSAREM (2044 subjects): 58% for AHI <15 (mild OSAREM: 31.5%, moderate OSAREM: 20%, severe OSAREM: 6.5%). REM-predominant OSA associated with PSG indices of poor sleep quality, but not with daytime sleepiness or QoL. |
| Mokhlesi [34], 2014 | Cross-sectional and longitudinal with follow-up 24 years; 4385 sleep studies in 1451 subjects (mean age 54 years, 46% women); ABPM studies in 742 subjects, Wisconsin Sleep Cohort | At least 30 min of REM sleep at PSG; AHIREM stratified by severity (<1, 1–4.9, 5–14.9 and ≥15 events·h−1); subsample of subjects with AHINREM <5 events·h−1 (n=1216) | Cross-sectional analysis: association of OSAREM with prevalent HT Longitudinal analysis: association of OSAREM with incident HT | Prevalence of OSAREM in the subsample of REM-predominant OSA: 33% of the total studies. Cross-sectional: significant association of AHIREM and prevalent HT, confirmed in the sample of patients with ABPM data; no association with AHINREM or supine position. AHIREM not associated with daytime sleepiness. Longitudinal: AHIREM >15 events·h−1 associated HT. Similar results in the sample with ABPM measurements. |
| Appleton [40], 2016 | Longitudinal; 837 nondiabetic men from the MAILES study | AHIREM: <10 events·h−1 no OSAREM; 10–19 events·h−1 mild OSAREM; 20–29 events·h−1 moderate OSAREM; >30 events·h−1 severe OSAREM | Association with prevalent and incident HbA1c >6% in 2010–2011 compared to 2002–2006 (MAILES 1) and 2007–2010 (MAILES 2) | No estimate of REM-predominant OSA. AHIREM not associated with either prevalent or incident HbA1c >6%. |
| Acosta-Castro [35], 2018 | Cross-sectional; 2074 subjects (mean age 57 years, 51.7% women), HypnoLaus Sleep cohort | At least 30 min of REM sleep at PSG; AHIREM according to four categories (>5, 5.0–9.9, 10–19.9 and ≥20 events·h−1) Analysis in the entire cohort and in patients with no–mild SDB (total AHI <10 events·h−1, n=1047) and with exclusive REM-SDB (AHINREM <10 events·h−1, n=1241) | Association of AHIREM with CV, metabolic and psychiatric comorbidities | Overall prevalence of AHIREM ≥20 events·h−1: 40.8%. Dose–response association of AHIREM with metabolic syndrome, but not with diabetes or depression. AHIREM ≥20 events·h−1 associated with systolic and diastolic blood pressure. In no–mild SDB, prevalence of AHIREM ≥20 events·h−1: 21.2%. AHIREM ≥20 events·h−1 associated with metabolic syndrome and diabetes. In exclusive REM-SDB, prevalence of AHIREM ≥20 events·h−1: 9.1%. Dose–response association of AHIREM with metabolic syndrome and diabetes. |
| Ljunggren [36], 2018 | Cross-sectional; 201 women, mean age 49.8 years, from the SHE study; exclusion of subjects with CV events or CPAP treatment in the previous 10 years | At least 30 min of REM sleep at PSG: severe OSAREM defined as AHIREM >30 events·h−1 Analysis in the subgroups with total AHI <15 events·h−1 and with total AHI <15 events·h−1+AHINREM <5 events·h−1 |
Association of OSAREM with carotid intima thickness | Increased carotid intima, but not media, thickness in women with severe OSAREM, even after multiple adjustments including blood pressure, lipid levels and diabetes. Apnoea duration but not hypoxic markers were associated with intima thickness. Similar results in the subgroups with total AHI <15 events·h−1 (n=139, 69% of the sample) and with total AHI <15 events·h−1+AHINREM <5 events·h−1 (n=99, 49% of the sample). |
| Aurora [41], 2018 | Longitudinal; 3265 subjects (mean age: 62 years, 63.1% women) with baseline AHINREM <5 events·h−1 (with prevalent CVD n=452, without prevalent CVD n=2813) followed for 9.5 years, SHHS | AHIREM categorised according to clinical cut-off points: <5 (normal), 5.0–14.9 (mild), 15.0–29.9 (moderate) and ≥30.0 events·h−1 (severe disease) | Composite CV end-point, i.e. occurrence of nonfatal or fatal events, including myocardial infarction, coronary artery revascularisation, congestive heart failure and stroke | Composite CV in 53.5% and 18.0% of participants with and without prevalent CVD at baseline. AHIREM >30 events·h−1 associated with higher risk, but only in patients with prevalent CVD at baseline. Marginal association with time spent at SpO2 <90% during REM sleep, no association with arousals. |
| Bikov [37], 2019 | Cross-sectional; 94 volunteers (mean age 49 years, 68% women) free from lipid-modifying medications | REM-dependent OSA: AHIREM ≥5 events·h−1 and AHINREM <5 events·h−1 | Relationship between OSAREM or OSANREM and lipid profile | OSA diagnosed in 41 subjects (21 mild, 13 moderate, seven severe). REM-dependent OSA in 17% of the sample, no differences in lipid profile compared to controls. |
| Aurora [38], 2020 | Longitudinal; 1908 subjects with OSAREM and AHINREM <5 at baseline (mean age 60.7 years, 64% women), with follow-up PSG study about 5 years after enrolment, and complete data from the SHHS | AHIREM >5 events·h−1 at baseline and at least 15 min of REM sleep both at baseline and follow-up PSG Analysis performed also with cut-off values for AHIREM >10 and >15 events·h−1 | Natural history or OSAREM during a median follow-up of 5.3 years Development of OSA in NREM sleep and determinants of progression Association with incident CVD | At baseline OSAREM in 44.9% of women and 46.1% of men. The majority of the sample did not show progression of AHINREM to values >5 events·h−1. OSA progression was associated with age and AHIREM in both men and women, while increased BMI was predictive only in men. Resolution of OSAREM during follow-up was associated with younger age, lower BMI at baseline and decreased BMI at follow-up. An increased risk for incident CV event was only shown in women with baseline AHIREM ≥5 events·h−1 with AHINREM ≥5 events·h−1 at follow-up. |
| Ljunggren [39], 2022 | Cross-sectional; 253 women (SHE study) and 338 age- and BMI-matched men (MUSTACHE study) | At least 30 min of REM sleep at PSG: Severe OSAREM defined as AHIREM >30 events·h−1 Analysis in the subgroups with total AHI <15 events·h−1 and with AHINREM <5 events·h−1 |
Association of OSAREM with carotid intima thickness | Prevalence of severe OSAREM: 26.6% in the entire sample, 37.9% in women and 18% in men. Mean AHIREM higher in women (17.6 events·h−1) than in men (6.5 events·h−1), minor differences in mean AHINREM (5.2 events·h−1 in women, 3.9 events·h−1 in men). Severe OSAREM associated with carotid intima thickness, persistent significance after adjustments. In sex-stratified analysis, significant association only in women. Subgroup analysis not significant but very small number of observations. |
AHI: apnoea–hypopnoea index; ABPM: ambulatory blood pressure; BMI: body mass index; CV: cardiovascular; CVD: cardiovascular disease; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes of Sleep Questionnaire; GDS: Geriatric Depression Scale-15; HbA1c: glycosylated haemoglobin; HT: hypertension; MAILES: Men Androgen Inflammation Lifestyle Environment and Stress; MrOS Sleep: Sleep Disorders in Older Men; MUSTACHE: Men in Uppsala; a Study of sleep, Apnea and Cardiometabolic Health; NREM: non-REM; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; QoL: quality of life; SDB: sleep-disordered breathing; SF-12: Short Form-12; SHE: Sleep and Health in Women; SHHS: Sleep Heart Health Study; SpO2: oxygen saturation measured by pulse oximetry; TST: total sleep time.
TABLE 3.
Studies on rapid eye movement (REM) obstructive sleep apnoea (OSA) in clinical samples
| First author [ref.], year | Study type; sample | Diagnostic criteria | Outcomes | Results |
| O’Connor [43], 2000 | Cross-sectional retrospective in 838 OSA patients (mean age: men 48.6 years; women 50.8 years; women 24.6%) | OSAREM: overall AHI between 10 and 25 events·h−1, AHIREM/AHINREM >2 and AHINREM <10 events·h−1 |
Sex-related differences in OSA | Prevalence of OSAREM: 24% in men, 62% in women. Lower overall AHI in women secondary to low AHINREM, AHIREM not different between sexes. Largest difference between men and women in the mild–moderate OSA range. No relationship with age in both men and women. |
| Resta [44], 2005 | Observational; 45 severely obese OSA patients (20 women age- and weight-matched to 25 men), mean age 44 years, BMI 40 kg·m−2 | OSAREM: overall AHI between 10 and 25 events·h−1, AHIREM/AHINREM >2 events·h−1 and AHINREM <10 events·h−1 | Sex-related differences in sleep and OSA in severely obese patients | Prevalence of OSAREM: 35% in women and 4% in men. Lower OSA severity and sleep efficiency and higher number of awakenings in patients with OSAREM. |
| Haba-Rubio [45], 2005 | Cross-sectional retrospective; 415 OSA patients (mean age 54.1 years, 73% men) | AHIREM/AHINREM >2 Exclusion criteria: 1) AHI ≤5 events·h−1 of TST; 2) previous treatment for SDB; 3) REM sleep <15% of TST during nocturnal recording |
Frequency and clinical characteristics of OSAREM including subjective (ESS) and objective (MWT, n=228) sleepiness | SDBREM in 36.4% of the sample (women: 46.4%, men 53.6%). No difference in symptoms or sleepiness between SDBREM and SDBNREM groups, but OSA more severe in patients with SDBNREM. Declining frequency of SDBREM from mild to severe OSA. SDBREM associated with higher BMI in mild–moderate OSA and more frequent in women except in severe OSA cases. |
| Koo [55], 2008 | Observational; 2486 OSA patients (mean age 50.8 years, 67.1% men) | At least 10 min of REM sleep at PSG OSAREM criteria: 1) overall AHI ≥5 events·h−1; 2) AHINREM <15 events·h−1; and 3) AHIREM/AHINREM ≥2 |
Sex-related differences in OSA | Prevalence of OSAREM: 21% in men, 40.8% in women. Patients with OSAREM were younger and showed longer REM sleep duration. Prevalence of OSAREM decreased with age and increasing BMI in both men and women. No difference in positional AHI was found for any sleep stage between sexes. |
| Koo [56], 2008 | Cross-sectional retrospective; 221 patients with OSAREM (mean age 50 years, 33.5% men) | OSAREM criteria: 1) age ≥18 years; 2) overall AHI ≥5 events·h−1; 3) AHINREM <15 events·h−1; 4) AHIREM/AHINREM >2; and 5) time spent in REM sleep >10 min | Effects of age and sex in OSAREM | Prevalence of OSAREM in the entire sample of OSA patients (n=1540): 14.4% (24.5% of women, 7.9% of men in the entire sample). Comorbidities: depression in 41.2%, at least one cardiovascular risk factor in 67.7% and EDS in 68.1%. In both men and women, OSAREM more frequent in patients younger than 55 years of age and directly related to BMI in women. |
| Pamidi [53], 2011 | Cross-sectional; 931 consecutive OSA patients (mean age 50 years, 44.4% men) | Overall AHI ≥5 events·h−1, with AHIREM/ AHINREM ≥2, AHINREM< 8 events·h−1 and REM duration >10.5 min | Association of OSAREM with subjective sleepiness (ESS) and QoL by SF-12 | Prevalence of OSAREM: 13.5%. OSAREM patients were younger and more often women (76.2 versus 52.4%) compared to nonstage-specific OSA patients. AHIREM did not predict sleepiness or QoL. Depressive symptoms and BMI predicted ESS and QoL in the OSAREM group. |
| Conwell [57], 2012 | Cross-sectional; 931 consecutive OSA patients (mean age 50 years, 44.4% men) | Definition 1: overall AHI ≥5 events·h−1 and AHIREM/AHINREM ≥2 Definition 2: overall AHI ≥5 events·h−1, AHIREM/ AHINREM ≥2 and AHINREM <15 events·h−1 Definition 3: overall AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, AHINREM <8 events·h−1 and at least 10.5 min of REM sleep duration |
Prevalence of OSAREM according to different definitions | Prevalence of OSAREM varied according to the definition used (1: 36.7%; 2: 24.4%; 3: 13.5%). OSAREM more prevalent in women (78 versus 48% in men), younger individuals (mean age 45 versus 52 years) and African Americans. Similar degrees of obesity and sleepiness, better sleep quality, and lower prevalence of diabetes and HT in OSAREM compared to patients with nonsleep stage-dependent OSA. |
| Sakao [42], 2015 | Cross-sectional; 468 patients with suspected OSA (mean age 54.9 years, 22.9% women) | Three definitions of OSAREM: I: overall AHI ≥5 events·h−1 and AHIREM/AHINREM ≥2 II: AHINREM <15 events·h−1 in addition to I III: AHINREM <8 events·h−1 and at least 10.5 min of REM sleep in addition to I |
Prevalence and features of OSAREM in Japanese subjects | Prevalence of OSAREM: 24.8% (I), 17.6% (II) and 11% (III). In women, prevalence of OSAREM increased from 33.6 to 40.1% from definition I to III and was higher than the prevalence of OSANREM. Subjects with OSAREM showed lower BMI and HbA1c levels than subjects with OSANREM. |
| Lee [46], 2016 | Cross-sectional retrospective; 1281 Korean OSA patients (mean age 54 years, 18% women) | Overall AHI ≥5 events·h−1, AHINREM <15 events·h−1 and AHIREM to AHINREM ratio >2 | Association of OSAREM with sleepiness (ESS), depressive symptoms (BDI) and health-related QoL (SF-36) | Prevalence of OSAREM: 18% (32.6% in women, 14.1% in men). OSAREM more frequent in mild–moderate than severe OSA. Significant association of OSAREM with depressive symptoms only in men. Sleepiness or QoL similar in OSAREM and non-REM related OSA groups. |
| Al Oweidat [54], 2018 | Cross-sectional; 478 Jordanian patients with OSA (mean age 55.3 years, 44.6% women) | Overall AHI ≥5 events·h−1 Broad definition of OSAREM: AHIREM/AHINREM ≥2; strict definition: AHINREM<5 events·h−1, AHIREM >5 events·h−1 and at least 30 min of REM sleep; OSANREM: AHIREM/AHINREM <2 |
Differences in demographic and PSG features between REM- and NREM-related OSA | Severe OSA in 72% of the sample. Prevalence of OSAREM: 18% (31% in women, 7.5% in men) according to the broad definition, 2.7% (5.2% in women, 0.8% in men) according to the strict definition. Higher arousal index and time spent at SpO2<90% and lower SpO2nadir in patients with OSANREM compared to patients with OSAREM for both broad and strict definitions. No differences in BMI, ESS and snoring between the two groups. |
| Mano [58], 2019 | Retrospective cross-sectional; 3234 Japanese OSA patients (mean age: 52.5 years, 14.5% women) | Three definitions of OSAREM: I: overall AHI ≥5 events·h−1 and AHIREM/AHINREM ≥2 II: AHINREM <15 events·h−1 in addition to I III: AHINREM <8 events·h−1 and at least 10.5 min of REM sleep in addition to I |
Effect of sex and age on OSAREM In women, analysis of the effects of menopause, defined as age >50 years | Overall prevalence of OSAREM 24.6%, 18.6% and 12.2% according to definitions I–III. In men, prevalence of OSAREM decreased with age, from 22.8 in men under 50 years to 19.1% in men over 50 years (definition I). Corresponding values in women were 44.3% under 50 years and 47.7% over 50 years (definition I). In multivariate analysis, adjustment for BMI and CT90 slightly decreased significance, whereas further adjustment for AHINREM strongly reduced the difference between sexes, both below and above age 50. |
| Bahammam [47], 2020 | Prospective observational; 2169 OSA patients (mean age: 46.7 years, 38% women) | Predominant OSAREM: overall obstructive AHI ≥5 events·h−1, AHINREM <15 events·h−1 and AHIREM/AHINREM ≥2. OSANSS: AHI ≥5 events·h−1, criteria for OSAREM not fulfilled | Analysis of clinical and sleep features, and of comorbidities | Prevalence of OSAREM: 17% (25% in women, 12% in men). OSAREM was more frequent at younger age but was unrelated to menopause at multivariate analysis. In OSAREM, frequent nocturnal chest pain, headache at awakening, nocturnal awakening with palpitations and higher prevalence of bronchial asthma, while snoring and overall prevalence of HT and ischaemic heart disease were lower than in OSANSS. In men, OSAREM was independently associated with younger age, HT, bronchial asthma, high sleep efficiency, low amount of NREM sleep stage 1 and lower AHI and SpO2 nadir. In women, OSAREM was independently associated with younger age, higher BMI, less HT, hypothyroidism, less sleepiness, low amount of NREM sleep stage 1 and lower amount of time spent at SpO2: <90%. |
| Sasai-Sakuma [48], 2021 | Retrospective cross-sectional; 1458 Japanese OSA patients (median age 48 years, 9.7% women) | Three definitions of OSAREM: I: overall AHI ≥5 events·h−1 and AHIREM/AHINREM ≥2 II: AHINREM <15 events·h−1 in addition to I III: AHINREM <8 events·h−1 and at least 10.5 min of REM sleep in addition to I |
Effects of gender and OSA severity on OSAREM prevalence | Prevalence of OSAREM (definition II) was 0% in severe OSA, 18.9% in moderate and 47% in mild OSA. Compared with OSANSS, patients with OSAREM showed higher BMI and female predominance, lower AHI and ODI, and higher sleep efficiency, but no difference in prevalence of daytime sleepiness or cardiometabolic comorbidities. |
| Chiu [49], 2022 | Retrospective cross-sectional; 1331 Korean OSA patients aged >20 years (median age 53 years, 20.5% women) | REM sleep: at least 30 min. OSAREM: AHIREM/AHINREM >2 and AHINREM <15 events·h−1; OSANREM: AHIREM/AHINREM ≤2; OSANSS: AHIREM/AHINREM >2 and AHINREM ≥15 events·h−1; isolated OSAREM: AHIREM >5 events·h−1 with AHINREM <5 events·h−1 | Clinical demographics, OSA-related symptoms, PSG results and medical comorbidities in OSAREM, OSANREM and OSANSS | Prevalence of OSAREM: 31.1%, OSANREM: 60.7%, OSANSS: 8.2%. OSAREM more frequent in women (53.1 versus 25.4% in men), mild–moderate than severe OSA based on overall AHI and associated with longer duration of desaturations and lower SpO2 nadir. No difference in ESS score or prevalence of comorbidities compared to the other groups. |
| Lee [50], 2022 | Retrospective cross-sectional; 692 Korean OSA patients (mean age 50.3 years, 28.2% women) | REM sleep: at least 30 min; OSAREM: overall AHI >5 events·h−1, AHIREM/AHINREM ≥2 and AHINREM <15 events·h−1 | Prevalence and clinical characteristics of OSAREM | Prevalence of OSAREM: 20.2% (38.4% of women, 13% of men). In the OSAREM group, females represented 53.6%. OSAREM present in 69.3% of patients with mild OSA and 30% of patients with moderate OSA. Prevalence of HT and diabetes lower in patients with OSAREM than in patients with OSANREM. |
| Sattaratpaijit [51], 2022 | Retrospective cross-sectional; 408 Thai OSA patients (mean age 49.7 years, 39.9% women) | At least 10.5 min of REM sleep; OSAREM: overall AHI ≥5 events·h−1, AHIREM/AHINREM >2 and AHINREM <15 events·h−1 | Prevalence and clinical characteristics of OSAREM | Prevalence of OSAREM: 21.6% (29.4% of women, 16.3% of men). OSAREM significantly associated with female sex (OR 2.35), age <60 years (OR 2.52) and mild OSA (OR 17.46). |
| Qanash [52], 2023 | Retrospective cross-sectional; 609 Saudi OSA patients (mean age 49 years, 42% women) | Strict definition: AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, AHINREM <15 events·h−1, at least 30 min of REM sleep Intermediate definition AHI ≥5 events·h−1, AHIREM/AHINREM ≥2, AHINREM <15 events·h−1 Lenient definition: AHI ≥5 events·h−1, AHIREM/AHINREM ≥2 |
Prevalence and clinical characteristics of OSAREM | Prevalence of OSAREM: 26% (women 36%, men 18%) according to the strict definition, 33% (women 48.4%, men 21.8%) according to the moderate definition and 52% (women 69.5%, men 35.4%) according to the lenient definition. Milder OSA and desaturation severity in OSAREM compared to OSANREM. No differences in ESS or prevalence of comorbidities between OSAREM and OSANREM. |
| Huang [61], 2023 | Cross-sectional; 4152 subjects with suspected OSA (mean age 49 years, 21% women), Shangai Sleep Health Study cohort | Stratification of the sample according to AHIREM in the no OSA and mild, moderate and severe OSA ranges Analysis in the subgroup with AHINREM <5 events·h−1 |
Association of OSAREM with cardiovascular risk, assessed as FRS and autonomic imbalance, assessed as HRV parameters in both subjects with and without prevalent CVD | Severe OSAREM associated with increased FRS, but not with prevalent CVD, in the multivariate analysis. High LF/HF, high LF and high HF associated with AHIREM in the subgroup with AHINREM<5 events·h−1. HRV parameters mediated the relationship between AHIREM and both prevalence of CVD and high FRS. |
AHI: apnoea–hypopnoea index; BDI: Beck Depression Inventory; BMI: body mass index; CT90: cumulative time spent at SpO2 <90%; CVD: cardiovascular disease; EDS: excessive daytime sleepiness; ESS: Epworth Sleepiness Scale; FRS: Framingham Risk Score; HbA1c: glycosylated haemoglobin; HF: high-frequency spectrum; HRV: heart rate variability; HT: hypertension; LF: low-frequency spectrum; MWT: maintenance of wakefulness test; ODI: oxygen desaturation index; NREM: non-REM; OR: odds ratio; OSANSS: nonstage-specific OSA; PSG: polysomnography; QoL: quality of life; SDB: sleep-disordered breathing; SF-12: Short Form-12; SF-36: Short Form-36; SpO2: oxygen saturation measured by pulse oximetry; TST: total sleep time.
Several studies, in both the general population and OSA patient cohorts, assessed the prevalence of REM OSA, its main clinical features and its possible prognostic implications. The majority of the studies are cross-sectional analyses of large cohorts, but longitudinal studies have provided information on the potential health risks associated with REM OSA and the evolution of REM OSA over time.
Studies in the general population
The results of cross-sectional studies highlight differences in prevalence rates, likely attributable to the different definitions of REM OSA and the variable sex distribution in the samples (table 2). Nevertheless, there is agreement on some aspects since REM OSA:
occurs with decreasing frequency from mild to severe OSA [33, 37];
does not appear to be associated with daytime sleepiness [32–34], poor quality of life [32, 33] or subjective insomnia [32];
does not seem to be associated with the supine position [34].
On the other hand, the data on sleep quality in OSA REM show variable results, since one study in elderly men reported an association with poor sleep quality [33], while other studies found no change in sleep structure/duration [34, 36, 37, 39]. Decreased total sleep time and low percentage of slow-wave sleep were only found in subjects with moderate–severe REM OSA [35].
As for the role of REM OSA with regard to comorbidities, systemic hypertension, evaluated either cross-sectionally or longitudinally, was shown to be associated with REM OSA, but not with NREM OSA [34]. Another study reported that moderate–severe REM OSA was associated with hypertension and metabolic abnormalities such as metabolic syndrome and diabetes, but not with depression [35]. No association was shown between REM OSA and either prevalent or incident increase in glycosylated haemoglobin (HBA1c) in men [40] or lipid profile [37]. Increased carotid intima thickness was reported in women, but not in men, with severe OSA in REM sleep [36, 39], suggesting a possible relationship of REM OSA with increased cardiovascular risk. In this regard, a longitudinal analysis in the Sleep Heart Health study cohort examined the impact of REM OSA on the occurrence of a composite cardiovascular end-point, i.e. fatal and nonfatal myocardial infarction, coronary revascularisation, congestive heart failure and stroke, in patients with or without prevalent cardiovascular disease. Isolated severe REM OSA was associated with the composite outcome only in patients with prevalent cardiovascular disease and correlated marginally with hypoxia during REM sleep [41].
An interesting question concerns the evolution of isolated REM OSA over time, since the occurrence of REM OSA might evolve towards a progressive extension to events in NREM sleep. In patients with isolated REM OSA, i.e. with NREM AHI <5 events·h−1 at baseline, progression of OSA in NREM sleep to an AHI >5 events·h−1 occurred in a minority of patients and was associated with age and REM AHI in both men and women, and with increased body mass index (BMI) only in men. On the other hand, regression of REM OSA was observed in younger nonobese patients at baseline, who showed a decrease in BMI during follow-up [38].
Studies in OSA patient cohorts
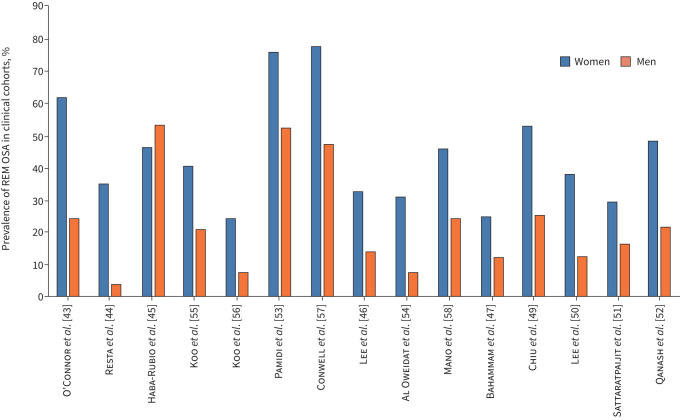
The studies in OSA patients are mainly retrospective cross-sectional analyses of existing cohorts (table 3). The prevalence rates of REM OSA according to sex (figure 1) were variable, possibly due to different definitions of REM OSA, variable sex distribution in the sample and different ethnicities of the OSA patients being examined. Nevertheless, REM OSA occurred more frequently in women than in men in all studies [42]. The results obtained in OSA patients confirmed the higher prevalence of REM OSA in mild–moderate than in severe OSA [43–52]. Sleep quality was quite preserved in patients with REM OSA compared to patients with NREM OSA, i.e. lower arousal index and/or lower percentage of stage 1 NREM sleep [45, 47, 50, 51, 53, 54], except in a study on severely obese OSA patients [44].
FIGURE 1.
Prevalence of rapid eye movement obstructive sleep apnoea (REM OSA) in clinical cohorts. Prevalence of REM OSA is reported in women (blue) and men (orange) for different studies. All studies, but one, found a higher prevalence of REM OSA in women than in men.
REM OSA frequently occurred in young to middle-aged patients [51, 53, 55–58] and the differences between men and women progressively decreased at older age [55]. The seminal study by O’Connor et al. [43], however, did not find any relationship between age and REM OSA in both sexes. Some studies addressed the question of the possible role of menopause. The study by Mano et al. [58] reported that the risk of REM OSA was higher in women aged >50 years. Conversely, Bahammam et al. [47] found a progressive decrease in the prevalence of REM OSA in ageing women; in multivariate analysis age remained significant, while menopausal status did not. Short-term hormone replacement in post-menopausal women with OSA did not affect overall OSA severity and decreased AHI in REM sleep nonsignificantly [59]. In a prospective study in women during the menopausal transition, respiratory events in REM sleep at baseline predicted hypertension, increased at follow-up and were independent of hormone replacement therapy use [60]. More data are necessary to understand the relative role of hormonal status and ageing in female REM OSA.
Although REM OSA usually occurs in the context of less severe OSA, i.e. lower overall AHI, compared to NREM OSA [45, 54], the frequency of daytime sleepiness was similar in patients with REM OSA and NREM OSA [45, 46, 48, 49, 52, 54, 57]. This finding is intriguing and would suggest that OSA REM is not as mild as it would seem. On the other hand, a recent study found evidence of abnormal autonomic modulation in severe REM OSA, suggesting a possible detrimental effect of REM OSA on cardiovascular risk [61]. Sympathetic activation in OSA is particularly prominent in REM sleep [62] and was found to be associated with excessive daytime sleepiness (EDS) [63–65]. These findings possibly account, at least partly, for the similar degree of sleepiness in REM OSA and NREM OSA.
Some of the differences in results may be accounted for by the characteristics of the patient samples and/or the definition of REM OSA used. Yet, the overall findings in patient cohorts seem to confirm the data in the general population, i.e. predominant or isolated REM OSA is present mainly in women and in young rather than old subjects (figure 1). Differences in BMI and symptoms between REM OSA and NREM OSA were not highly relevant and, when present, they could be at least partly explained by the usually worse severity of NREM OSA compared to REM OSA.
REM OSA in other diseases
REM OSA has been reported to occur in the context of other diseases. Adult narcoleptic patients with OSA were older and had a higher BMI than patients without OSA and showed a high prevalence of REM OSA independent of the presence of cataplexy [66].
REM sleep behaviour disorder (RBD) is characterised by loss of general muscle atonia during REM sleep. Attention has been drawn to the possibility that RBD may protect against REM OSA because UA muscle tone is usually preserved in RBD. On the other hand, sleep apnoea-induced movement arousals accompanied with vocal sounds may mimic features of RBD [67]. In a case–control study, 109 patients with idiopathic RBD and OSA (RBD-OSA) were consecutively enrolled and compared with matched OSA controls without RBD. In the latter group, the AHI was significantly higher during REM than during NREM sleep (p<0.01). However, in RBD-OSA patients, the AHI was significantly lower during REM sleep as compared with non-RBD controls, the prevalence of REM OSA being 9.2 versus 33.0%, respectively [68]. Another study showed significantly less oxygen desaturation during REM sleep in RBD-OSA patients in comparison with non-RBD controls [69]. In proven RBD with associated OSA, symptoms of RBD may improve under CPAP therapy [70]. Thus, the relation between RBD and REM OSA may be reciprocal, as RBD may protect against REM OSA and treatment of OSA may alleviate RBD symptoms. More extensive research is needed, however, to corroborate this hypothesis.
A retrospective study in untreated patients with allergic rhinitis, sensitised to house dust mites (HDMs) or other allergens, found that the overall AHI or respiratory disturbance index (RDI) was on average low and similar to that recorded in controls. However, the REM RDI was higher in patients with rhinitis than in nonallergic subjects [71] and highest in patients with HDM allergy. Although sleep quality was shown to improve after long-term treatment for allergic rhinitis [72], no study to date has objectively recorded sleep in patients treated for allergic rhinitis.
Prevalence of REM OSA was assessed in a cohort of patients with coronary artery disease (CAD). The results were similar to those recorded in non-CAD cohorts, since the prevalence of REM OSA was 25.5% (26% in women versus 9.9% in men) and more frequent in obese than nonobese patients (42.5 versus 24.4%). The Mental Component Summary of the Short-Form 36 Assessment of Quality of Life was the only outcome significantly and inversely correlated with REM AHI [73]. Another study in patients with percutaneous coronary intervention within the previous 6–36 months found an 81% prevalence of REM OSA, positively associated with BMI, systolic BP and diabetes mellitus at univariate analysis, whereas only diabetes was a significant predictor of REM OSA at multivariate analysis [74].
It is likely that the prevalence of REM OSA could be significant in other conditions besides the ones analysed, but further studies are needed. The difficulty in obtaining data on REM OSA is linked to the widespread use of cardiorespiratory polygraphy, which can only suggest occurrence of REM OSA without any objective evidence. More data will be provided by sleep recordings over several nights, since significant variability in OSA, especially in the mild–moderate range, has been shown over different nights [75, 76].
Clinical manifestations
EDS
In OSA patients, the severity of the disorder, graded by the AHI or arousal index, only explains a small part of the variance of EDS assessed by the multiple sleep latency test [77, 78]. Studies in clinical OSA patients comparing the relative contribution of NREM versus REM OSA were unable to identify any relevant additional impact of the latter when EDS was measured either subjectively [79] or objectively [80]. In experimentally induced REM OSA (by withdrawing CPAP therapy exclusively during REM sleep), the results of mean reaction time and psychomotor vigilance tests were not significantly changed [81]. Moreover, REMp OSA did not seem to affect EDS differently from NREM OSA in the general population. This lack of significance has been observed in cross-sectional studies in adults [32, 48] and in a prospective study in children [82]. Thus, evidence to suggest that REMp or REMi OSA independently impairs daytime vigilance is lacking.
Insomnia
Whether REMp or REMi OSA may show stronger associations with insomnia than NREM or nonsleep-stage-dependent OSA has not been studied extensively. Only one study using the Pittsburgh Sleep Quality Index (PSQI) addressed this issue and found that REM-related OSA was significantly associated with an increased PSQI in all adjusted models [83]. In the subgroup analysis, the coefficients of all models were higher in female than in male patients with REM-related OSA. Because insomnia is much more prevalent in women, sex may be an important factor, potentially confounding the interpretation of these results [84]. Further research is awaited to clarify the effects of REM OSA on insomnia symptoms. Furthermore, better tools to specifically assess insomnia should be applied in this area of investigation.
Anxiety and depression
Studies investigating how REM OSA relates to anxiety and depression are also scarce. An observational study comparing healthy controls (n=18), patients with nonsleep-stage-dependent OSA (n=18) and REMi OSA patients (n=17) found significant differences in results from the Profile of Mood State test (43.8±2.6, 51±4.0 and 65.6±7.7, respectively; p=0.019) [85]. From this small sample of subjects, it was inferred that REMi OSA could be deleterious for emotional health. In an observational study of 72 patients with mild REMi OSA and 94 patients with mild NREM OSA, anxiety and depression scores were significantly higher in the former group. In a large sample of 1281 consecutively recruited OSA patients, linear regression analysis showed that the presence of REMp OSA was significantly associated with higher scores on the Beck Depression Inventory questionnaire [46]. Remarkably, this association was only present in men, not in women. Furthermore, no significant associations could be demonstrated between REM OSA and indices of anxiety or depression in older men from the general population [33] or in a clinical sample of OSA patients [86]. To summarise, studies on the association between REM OSA and anxiety/depression are hampered by methodological limitations and are overall inconclusive.
Arterial hypertension
Associations between elevated BP and REM OSA, but not NREM OSA, were documented in different cross-sectional and prospective general population studies using home polysomnography.
In the Wisconsin Sleep Cohort, the REM AHI, either defined as a categorical or continuous variable, had predictive value for prevalent hypertension, after controlling for common confounding factors [34]. This was also the case in subjects with REMi OSA, in whom the NREM AHI was below 5 events·h−1. In contrast, NREM AHI did not predict hypertension in any of the models. A separate prospective study in the same cohort revealed that there was a greater risk of developing systolic and diastolic nondipping BP with greater severity of REM OSA in a dose-dependent manner [87]. When controlling for NREM AHI and other covariates, subjects with REM AHI ≥15 events·h−1 showed a higher relative risk of incident systolic and diastolic nondipping than those with REM AHI <1 event·h−1.
In the Men Androgens Inflammation Lifestyle Environment and Stress (MAILES) study, longitudinal and cross-sectional associations of previously unrecognised OSA with hypertension were examined. Severe REM OSA (AHI ≥30 events·h−1) was associated independently with prevalent and recent-onset hypertension [40]. In addition, in subjects not considered to have OSA (AHI <10 events·h−1), an REM AHI ≥20 events·h−1 was significantly associated with prevalent hypertension. The relationship with recent-onset hypertension was positive but not significant. No such associations with the NREM AHI were seen.
Data from the population-based HypnoLaus Sleep Cohort (48.3% men, mean age 57±11 years) were analysed to assess REM AHI versus NREM AHI as predictors for prevalent hypertension and other common diseases. The prevalence of REM AHI ≥20 events·h−1 was 40.8% in the entire cohort. Systolic and diastolic BP were positively associated with REM OSA, defined as REM AHI ≥20 events·h−1.
Associations between REM OSA and hypertension have also been demonstrated in patient populations. One study assessed REM AHI and morning versus evening BP levels in a patient sample [88]. The probability of morning hypertensive BP levels was significantly and independently associated with age, BMI and REM AHI, but not NREM AHI. REM AHI was not associated with evening hypertensive BP levels. In a recent study in a cohort with >10 000 OSA patients, fully adjusted models demonstrated a significant dose–response relationship between arousal index during REM sleep (REM AI) and prevalent hypertension [89]. The highest odds ratio of prevalent hypertension was found in a subgroup with an REM AI >40 events·h−1. On the contrary, NREM AI did not predict hypertension in any model. However, in another study on OSA in patients with type 2 diabetes, a statistically significant association was found between NREM OSA and hypertension, which was not the case for REM OSA [90].
To summarise, associations between REM OSA and prevalent hypertension have been demonstrated in both general and clinical population studies. Thus, an increased REM AHI could be clinically relevant even in the presence of a global AHI <10 events·h−1 or an REMi OSA condition (defined by an NREM AHI <5 events·h−1). Contemporary guidelines recommend that CPAP therapy should be used at least 4 h per night [91]. However, if CPAP therapy is limited to the first half of the sleep period, most of the nocturnal REM sleep will be left untreated. Therefore, REM OSA would require that CPAP treatment should be used during the entire sleep period.
Cardiovascular and metabolic complications
Besides its link with hypertension, REM OSA may also be implicated in cardiovascular and metabolic comorbidities. In a sample of the Sleep Heart Health Study (SHHS) [41], the incidence of cardiovascular events was assessed in subjects with and without prevalent cardiovascular disease during an average follow-up of 9.5 years. In stratified analyses, the postulated association could be confirmed only in those with prevalent cardiovascular disease and severe REM OSA, with an adjusted hazard ratio of 2.56 (95% CI 1.46–4.47). In another follow-up survey using SHHS data, it was evaluated whether subjects with REMi OSA would also develop NREM OSA in the course of time. Also, it was assessed whether the development of OSA during NREM sleep was associated with incident cardiovascular disease [38]. The majority of the participants did not develop OSA during NREM sleep. However, the likelihood of progression of OSA into NREM sleep did increase with higher baseline REM AHI. Obesity was a significant contributing factor. The relative risk for incident cardiovascular events among those who developed an NREM AHI of ≥5 events·h−1 at the follow-up visit was elevated only in women with REMi OSA at baseline. Another study, however, showed different results indicating that REM OSA could not be related to an increased cardiovascular risk. REM OSA was identified as a separate entity from a cluster analysis study of a multisite, observational US veteran cohort [92]. These findings indicate that differences in population samples and applied methodology may affect risk assessment in REM OSA patients.
It is known that OSA may compromise glycaemic health. OSA has been implicated as an independent risk factor for insulin resistance in subjects who may not be obese [93, 94]. Furthermore, the degree of OSA-related hypoxaemia seems to correlate with HbA1c levels in subjects without diagnosed diabetes [95]. The question whether REM OSA by itself may worsen glucose metabolism has been addressed in a limited number of studies. The naturally occurring decline of interstitial glucose concentrations during sleep was reversed in REM OSA, whereas NREM OSA had no appreciable effect [96]. In a prospective study of obese type 2 diabetes patients, incremental quartiles of REM AHI were significantly associated with increasing levels of HbA1c after adjusting for common confounders [97]. Associations between REM OSA and insulin resistance have also been investigated in the SHHS. The REM AHI was significantly associated with increasing levels of insulin resistance after controlling for common confounders. In this study, however, NREM AHI also played a role as it correlated significantly with fasting and post-prandial glucose levels [98].
A recent study demonstrated that correlations between lipid profiles and REM AHI versus NREM AHI were no longer present after adjustment for BMI. Furthermore, there was no difference in the lipid profile of REM OSA subjects as compared with healthy controls [37].
Considering the effects on hypertension and cardiometabolic disorders, there may be a causal role for REM OSA in the development of the metabolic syndrome. Preliminary evidence seems to confirm this hypothesis, as REM OSA was shown to be significantly associated with key features of this syndrome, independently of the effect of covariates such as age and obesity [99].
Treatment
CPAP therapy
Adequate compliance with CPAP therapy is important to prevent REM OSA. CPAP use for at least 6 h per night (thus also covering REM sleep in the second half of the sleep period) is associated with a reduction in the incidence of cardiovascular events. This has been shown in an observational study in 5138 OSA patients who were treated with long-term CPAP therapy followed for a median of 6.6 years [100, 101].
Some studies addressed the question of CPAP effectiveness in REM OSA and compliance to treatment. In the series by Conwell et al. [57], CPAP titration was recommended in 88% of patients with REM OSA, but only 66% of them underwent titration of fixed CPAP and 30-day compliance was available in 27% of the sample. Although no difference in compliance was observed compared to patients with NREM OSA, the large majority of patients with REM OSA did not accept, or comply with, CPAP treatment. In the study by Su et al. [102], CPAP treatment appeared equally effective in patients with REM (n=130) and NREM OSA (n=200), with objective data available in 68 and 65% of the patients, respectively. Automatic CPAP was more often prescribed to patients with REM OSA and average pressure was lower in patients with REM OSA compared to patients with NREM OSA. Changes in functional outcome measures were similar in the two groups after treatment.
More recent studies reported lower CPAP use in patients with REM OSA compared to patients with NREM OSA. Almeneessier et al. [103] prospectively followed 175 OSA patients, 30 of them with REM OSA, at 1, 6 and 12 months, and documented lower CPAP use at 1 year in patients with REM OSA compared to the rest of the sample (3.8 versus 5.1 h·night−1). Side-effects such as mask discomfort, facial skin irritation and nasal congestion were reported more often by patients with REM OSA (86.7 versus 35.2%). Good and partial adherence were recorded in 23.3 and 56.7%, respectively, of patients with REM OSA at 1 year, and adherence was lower than in NREM OSA at all time points. Hoshino et al. [104] found that a diagnosis of REM OSA was a strong predictor of drop-out from CPAP therapy. In the study by Hu et al. [105], only 48% of REM OSA patients prescribed CPAP were adherent to treatment after 1 year and showed decreased sleepiness and improved quality of life.
Non-CPAP treatments
Since patients with REM OSA poorly accept CPAP treatment, the clinical dilemma whether or not to treat is still an open problem [106]. Choosing a treatment other than CPAP may be appropriate since OSA REM often occurs in the mild–moderate AHI range. Effectiveness of oral appliances was similar in REM and NREM OSA, and the success rate was negatively influenced by BMI, especially in REM OSA patients [107]. REM OSA was successfully treated in a middle-aged woman by use of an intra-oral neuromuscular stimulation device [108]. It is possible that non-CPAP treatment of REM OSA may obtain better results than CPAP, but a lack of studies to date prevents any conclusion.
There are at present no published data on the effects of drugs specifically prescribed for the treatment of REM OSA.
Lifestyle interventions
Some studies suggest that lifestyle interventions may be useful in the treatment of REM OSA. Exercise and educational measures over 8 weeks did not affect overall AHI in patients with moderate–severe OSA, but decreased AHI in REM sleep, daytime sleepiness and body weight [109]. A 4-year follow-up study in patients with type 2 diabetes and REM OSA indicated that weight reduction obtained by lifestyle interventions was significantly related to changes in HbA1c. However, reductions in REM AHI (or NREM AHI) were not associated with improved glycaemic control in diabetic patients with OSA [110]. These results indicate that the role of confounders must be considered in treatment outcome studies on REM OSA.
Discussion
In summary, predominant or isolated REM OSA occurs frequently, especially in women with OSA in the mild–moderate AHI range and in younger patients. REM OSA has been convincingly associated with systemic hypertension, especially when the REM AHI is >20 events·h−1, both in cross-sectional and longitudinal studies. REM OSA does not always worsen over time and progression of OSA to NREM sleep has only been found in women with severe REM OSA and obesity at baseline.
Since OSA shows major sex-related differences, it is currently difficult to ascertain whether the relative contribution of different factors implied in REM OSA might be due to sex-related differences or if the features of REM OSA are independent of sex. Obesity appears to play a role in REM OSA, but the possible sex-related effects of the central versus peripheral distribution of adipose tissue in REM OSA are still unknown. Similarly, insomnia and depression occur more often in women than in men. Large population studies have reported sex-related differences but neither the prevalence of hypertension nor other outcomes have been analysed according to sex [34, 35, 41]. Therefore, the independent role of REM OSA should be explored in studies specifically designed to address these outcomes.
Current areas of uncertainty regard cardiometabolic changes and prognosis in predominant or isolated REM OSA. More long-term longitudinal studies are needed to clarify this question. The feasibility and effects of CPAP treatment remain a major issue in the management of patients with REM OSA. Currently, if a patient with REM OSA is symptomatic for sleepiness, CPAP treatment appears indicated and could be accepted. However, the high rate of treatment interruption or insufficient adherence would suggest that efforts to identify specific phenotypes of REM OSA with good CPAP adherence are necessary. Nevertheless, in the case of REM OSA, CPAP use for 4 h·night−1 would be insufficient, given the occurrence of REM sleep in the early morning hours [97]. On the other hand, very little evidence is available to indicate the effectiveness of alternative treatments or lifestyle interventions, which need to be verified by clinicians on a case-to-case basis.
Animal models do not appear useful to increase our knowledge on REM OSA. Obstructive apnoeas naturally occur in REM sleep in mice, especially in a model of Down syndrome [111]. However, reliable polysomnography signals during sleep are hard to obtain in rodents [112]. The widely used OSA model of intermittent hypoxia exposure in mice is known to decrease REM sleep compared to sleep fragmentation or control conditions [113]. The English bulldog could be another natural model of REM OSA anatomy [114], but it is uncertain if research in these animals is still ongoing.
The major limitation emerging from our analysis of the literature is the already underlined lack of standardisation of the definition for REM OSA: “Given the significant heterogeneity in defining REM-related OSA, it is not surprising that its epidemiology, natural history, and clinical significance are not well defined” [6]. Moreover, differences in the methodology used for the assessment of the AHI may have a significant impact on REM OSA case-finding [115].
Posture-dependent occurrence of apnoeas and hypopnoeas is frequent in OSA patients. Most often, the supine posture predisposes to sleep-disordered breathing, whereas the nonsupine position may alleviate obstructive breathing. In an observational study, supine-only OSA was common, occurring in 23–63% of a standard OSA population, whereas REMi OSA was much less common (approximately 10%). Supine-only OSA showed a greater impact on the overall severity of OSA than REMi OSA [116]. Both sleep state and postural mechanisms may affect the appearance and frequency of respiratory events. These effects should not be considered independently as they may, together, determine the severity of OSA [117]. Most publications on REM OSA, while requiring a recorded REM sleep time of at least 30 min, do not report data on position dependency. Studies based on polysomnography in which sufficient REM sleep is recorded in both the supine and nonsupine postures are scarce. Controlling for the confounding effect of body position on the phenotypic aspects of REM OSA implies the implementation of new studies with a sufficiently powered design.
In the vast majority of studies, the definition of REM OSA is based on the AHI, for which values are increased in this sleep state as compared with NREM sleep. Knowing that the AHI is an unprecise metric and a poor measure of clinical correlates of OSA, this approach may be misleading [118]. Discrimination between REM and NREM OSA should also consider the type of respiratory events (apnoeas versus hypopnoeas), their duration, as well as the concomitant systemic effects. The degree of hypoxaemia that is associated with respiratory events is an important systemic effect. The compilation of all hypoxaemic events during sleep, commonly known as the “hypoxic burden”, has been shown to better predict cardiovascular outcomes that the mere AHI [119, 120]. Novel indices of OSA severity should be utilised to assess the postulated discrepancies between REM and NREM OSA in terms of clinical manifestations and treatment outcomes.
Similar to Janus of the Roman myth, REM OSA appears double-faced. On one hand, it could be a rather benign form of OSA, due to its mild–moderate severity and the scarce tendency to progression. On the other hand, it could be symptomatic like NREM OSA, at least in terms of sleepiness, and be associated with cardiometabolic consequences, at least in some patient subgroups. This, coupled with the need for prolonged nightly treatment and the reported poor adherence to CPAP, leaves unsolved the clinical dilemma of REM OSA management. More well-designed studies, including testing of alternative non-CPAP treatments, are definitely needed. Prior to this endeavour, however, the sleep medicine community should decide on a standardised working definition of REM OSA. This would have to include not only the frequency and type of respiratory events, but also their systemic effects (e.g. the degree of hypoxaemia) and the loss (or preservation) of positional dependency. Differences in end-organ impact between NREM and REM OSA will only become clear if NREM and REM OSA can be described in unequivocal pathophysiological concepts.
Questions for future research
Reach a unified definition of REM OSA, allowing comparisons of results obtained by different studies.
Assess the contribution of sex-related features of OSA to the epidemiology, clinical presentation and outcomes of REM OSA.
Understand the role of REM OSA in the pathogenesis of daytime sleepiness and hypertension.
Include hypoxic and other markers of OSA severity beyond the usual AHI-based definition.
Address the potential of non-CPAP treatment in predominant or isolated REM OSA.
Acknowledgements
The authors thank Vanessa Lo Nano (PROMISE Department, University of Palermo, Palermo, Italy) for secretarial help.
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Polytarchou A, Moudaki A, Van de Perck E, et al. An update on diagnosis and management of obstructive sleep apnoea in the first 2 years of life. Eur Respir Rev 2024; 33: 230121.
Number 2 in the Series “Sleep and Breathing Conference 2023” Edited by Maria R. Bonsignore, Dirk A. Pevernagie and Refika Ersu
Conflict of interest: M.R. Bonsignore reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for Bioprojet and Jazz, outside the submitted work; participation on a Data Safety Monitoring Board or Advisory Board for Bioprojet, outside the submitted work; and is a current editorial board member for the European Respiratory Review. E. Mazzuca, P. Baiamonte, B. Bouckaert, W. Verbeke and D.A. Pevernagie have nothing to disclose.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, et al. . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019; 7: 687–698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest 1985; 87: 432–436. doi: 10.1378/chest.87.4.432 [DOI] [PubMed] [Google Scholar]
- 3.Series F, Cormier Y, La Forge J. Influence of apnea type and sleep stage on nocturnal postapneic desaturation. Am Rev Respir Dis 1990; 141: 1522–1526. doi: 10.1164/ajrccm/141.6.1522 [DOI] [PubMed] [Google Scholar]
- 4.Kass JE, Akers SM, Bartter TC, et al. . Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med 1996; 154: 167–169. doi: 10.1164/ajrccm.154.1.8680674 [DOI] [PubMed] [Google Scholar]
- 5.Pirzada AR, BaHammam AS. Rapid eye movement predominant obstructive sleep apnoea: prognostic relevance and clinical approach. Curr Opin Pulm Med 2021; 27: 514–522. doi: 10.1097/MCP.0000000000000817 [DOI] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep 2012; 35: 5–7. doi: 10.5665/sleep.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peker Y. REM sleep: a nightmare for patients with obstructive sleep apnea? Am J Respir Crit Care Med 2014; 190: 1088–1090. doi: 10.1164/rccm.201410-1897ED [DOI] [PubMed] [Google Scholar]
- 8.Nishimura A, Kasai T, Kikuno S, et al. . Apnea hypopnea index during rapid eye movement sleep with diabetic retinopathy in patients with type 2 diabetes. J Clin Endocrinol Metab 2019; 104: 2075–2082. doi: 10.1210/jc.2018-00946 [DOI] [PubMed] [Google Scholar]
- 9.Uchida T, Nishimura A, Kasai T, et al. . Relationship between obstructive sleep apnoea during rapid eye movement sleep and metabolic syndrome parameters in patients with type 2 diabetes mellitus. Sleep Breath 2021; 25: 309–314. doi: 10.1007/s11325-020-02129-7 [DOI] [PubMed] [Google Scholar]
- 10.Karuga FF, Kaczmarski P, Bialasiewicz P, et al. . REM-OSA as a tool to understand both the architecture of sleep and pathogenesis of sleep apnea–literature review. J Clin Med 2023; 12: 5907. doi: 10.3390/jcm12185907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silber MH, Ancoli-Israel S, Bonnet MH, et al. . The visual scoring of sleep in adults. J Clin Sleep Med 2007; 3: 121–131. doi: 10.5664/jcsm.26814 [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S, Drager LF, Pevernagie DA, et al. . Sleep and cardiovascular disease: heart failure, coronary artery disease, arrhythmias, and hypertension. In: Bassetti CL, McNicholas WT, Paunio T, et al., eds. Sleep Medicine Textbook. 2nd Edn. Regensburg, European Sleep Research Society, 2021; pp. 771–787. [Google Scholar]
- 13.Redline S, Budhiraja R, Kapur V, et al. . The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med 2007; 3: 169–200. doi: 10.5664/jcsm.26818 [DOI] [PubMed] [Google Scholar]
- 14.Xie A. Effect of sleep on breathing – why recurrent apneas are only seen during sleep. J Thorac Dis 2012; 4: 194–197. doi: 10.3978/j.issn.2072-1439.2011.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas NJ, White DP, Weil JV, et al. . Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis 1982; 125: 286–289. doi: 10.1164/arrd.1982.125.3.286 [DOI] [PubMed] [Google Scholar]
- 16.Douglas NJ, White DP, Weil JV, et al. . Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982; 126: 758–762. doi: 10.1164/arrd.1982.126.5.75 [DOI] [PubMed] [Google Scholar]
- 17.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med 2009; 180: 788–793. doi: 10.1164/rccm.200905-0773OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo P, Gartman EJ, Sethi JM, et al. . End-expiratory lung volume decreases during REM sleep despite continuous positive airway pressure. Sleep Breath 2020; 24: 119–125. doi: 10.1007/s11325-019-01857-9 [DOI] [PubMed] [Google Scholar]
- 19.Koo P, Gartman EJ, Sethi JM, et al. . Change in end-expiratory lung volume during sleep in patients at risk for obstructive sleep apnea. J Clin Sleep Med 2017; 13: 941–947. doi: 10.5664/jcsm.6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepard JW, Jr. Cardiopulmonary consequences of obstructive sleep apnea. Mayo Clin Proc 1990; 65: 1250–1259. doi: 10.1016/S0025-6196(12)62749-9 [DOI] [PubMed] [Google Scholar]
- 21.Hedner JA, Wilcox I, Laks L, et al. . A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis 1992; 146: 1240–1245. doi: 10.1164/ajrccm/146.5_Pt_1.1240 [DOI] [PubMed] [Google Scholar]
- 22.Eckert DJ, White DP, Jordan AS, et al. . Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013; 188: 996–1004. doi: 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 1990; 142: 295–300. doi: 10.1164/ajrccm/142.2.295 [DOI] [PubMed] [Google Scholar]
- 24.Vandenbussche NL, Overeem S, van Dijk JP, et al. . Assessment of respiratory effort during sleep: esophageal pressure versus noninvasive monitoring techniques. Sleep Med Rev 2015; 24: 28–36. doi: 10.1016/j.smrv.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 25.Krieger J, Sforza E, Boudewijns A, et al. . Respiratory effort during obstructive sleep apnea: role of age and sleep state. Chest 1997; 112: 875–884. doi: 10.1378/chest.112.4.875 [DOI] [PubMed] [Google Scholar]
- 26.Joosten SA, Landry SA, Wong AM, et al. . Assessing the physiologic endotypes responsible for REM- and NREM-based OSA. Chest 2021; 159: 1998–2007. doi: 10.1016/j.chest.2020.10.080 [DOI] [PubMed] [Google Scholar]
- 27.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med 2013; 187: 311–319. doi: 10.1164/rccm.201209-1654OC [DOI] [PubMed] [Google Scholar]
- 28.McSharry DG, Saboisky JP, Deyoung P, et al. . Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep 2014; 37: 561–569. doi: 10.5665/sleep.3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carberry JC, Jordan AS, White DP, et al. . Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep 2016; 39: 511–521. doi: 10.5665/sleep.5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckert DJ, Malhotra A, Lo YL, et al. . The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 2009; 135: 957–964. doi: 10.1378/chest.08-2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messineo L, Eckert DJ, Taranto-Montemurro L, et al. . Ventilatory drive withdrawal rather than reduced genioglossus compensation as a mechanism of obstructive sleep apnea in REM sleep. Am J Respir Crit Care Med 2022; 205: 219–232. doi: 10.1164/rccm.202101-0237OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chami HA, Baldwin CM, Silverman A, et al. . Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med 2010; 181: 997–1002. doi: 10.1164/rccm.200908-1304OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan A, Harrison SL, Kezirian EJ, et al. . Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in Osteoporotic Fractures in Men (MrOS) Sleep Study. J Clin Sleep Med 2013; 9: 191–198. doi: 10.5664/jcsm.2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokhlesi B, Finn LA, Hagen EW, et al. . Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med 2014; 190: 1158–1167. doi: 10.1164/rccm.201406-1136OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acosta-Castro P, Hirotsu C, Marti-Soler H, et al. . REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J 2018; 52: 1702484. doi: 10.1183/13993003.02484-2017 [DOI] [PubMed] [Google Scholar]
- 36.Ljunggren M, Lindberg E, Franklin KA, et al. . Obstructive sleep apnea during rapid eye movement sleep is associated with early signs of atherosclerosis in women. Sleep 2018; 41: zsy099. doi: 10.1093/sleep/zsy099 [DOI] [PubMed] [Google Scholar]
- 37.Bikov A, Lazar Z, Horvath P, et al. . Association between serum lipid profile and obstructive respiratory events during REM and non-REM sleep. Lung 2019; 197: 443–450. doi: 10.1007/s00408-019-00195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aurora RN, McGuffey EJ, Punjabi NM. Natural history of sleep-disordered breathing during rapid eye movement sleep. Relevance for incident cardiovascular disease. Ann Am Thorac Soc 2020; 17: 614–620. doi: 10.1513/AnnalsATS.201907-524OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ljunggren M, Naessen T, Theorell-Haglow J, et al. . Rapid eye movement sleep apnea and carotid intima thickness in men and women: a SHE-MUSTACHE cohort study. J Sleep Res 2022; 31: e13599. doi: 10.1111/jsr.13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appleton SL, Vakulin A, Martin SA, et al. . Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest 2016; 150: 495–505. doi: 10.1016/j.chest.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 41.Aurora RN, Crainiceanu C, Gottlieb DJ, et al. . Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med 2018; 197: 653–660. doi: 10.1164/rccm.201706-1112OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakao S, Sakurai T, Yahaba M, et al. . Features of REM-related sleep disordered breathing in the Japanese population. Intern Med 2015; 54: 1481–1487. doi: 10.2169/internalmedicine.54.4248 [DOI] [PubMed] [Google Scholar]
- 43.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161: 1465–1472. doi: 10.1164/ajrccm.161.5.9904121 [DOI] [PubMed] [Google Scholar]
- 44.Resta O, Carpagnano GE, Lacedonia D, et al. . Gender difference in sleep profile of severely obese patients with obstructive sleep apnea (OSA). Respir Med 2005; 99: 91–96. doi: 10.1016/j.rmed.2004.05.014 [DOI] [PubMed] [Google Scholar]
- 45.Haba-Rubio J, Janssens JP, Rochat T, et al. . Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest 2005; 128: 3350–3357. doi: 10.1378/chest.128.5.3350 [DOI] [PubMed] [Google Scholar]
- 46.Lee SA, Paek JH, Han SH. REM-related sleep-disordered breathing is associated with depressive symptoms in men but not in women. Sleep Breath 2016; 20: 995–1002. doi: 10.1007/s11325-016-1323-2 [DOI] [PubMed] [Google Scholar]
- 47.Bahammam RA, Al-Qahtani KM, Aleissi SA, et al. . The associations of gender, menopause, age, and asthma with REM-predominant obstructive sleep apnea: a prospective observational study. Nat Sci Sleep 2020; 12: 721–735. doi: 10.2147/NSS.S275051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasai-Sakuma T, Kayaba M, Inoue Y, et al. . Prevalence, clinical symptoms and polysomnographic findings of REM-related sleep disordered breathing in Japanese population. Sleep Med 2021; 80: 52–56. doi: 10.1016/j.sleep.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 49.Chiu HY, Liu YY, Shiao TH, et al. . Clinical characteristics of rapid eye movement-related obstructive sleep apnea: an experience in a tertiary medical center of Taiwan. Nat Sci Sleep 2022; 14: 1521–1532. doi: 10.2147/NSS.S368659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SC, Kim DE, Hwangbo Y, et al. . Does REM sleep-dependent obstructive sleep apnea have clinical significance? Int J Environ Res Public Health 2022; 19: 14147. doi: 10.3390/ijerph192114147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sattaratpaijit N, Kulalert P, Wongpradit W. Characteristics of rapid eye movement-related obstructive sleep apnea in Thai patients. Sci Rep 2022; 12: 11360. doi: 10.1038/s41598-022-13382-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qanash S, Mufti H, Alhejaili F, et al. . The prevalence of rapid eye movement-related obstructive sleep apnea in a sample of Saudi population. Ann Thorac Med 2023; 18: 90–97. doi: 10.4103/atm.atm_388_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pamidi S, Knutson KL, Ghods F, et al. . Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep Med 2011; 12: 827–831. doi: 10.1016/j.sleep.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 54.Al Oweidat K, AlRyalat SA, Al-Essa M, et al. . Comparing REM- and NREM-related obstructive sleep apnea in Jordan: a cross-sectional study. Can Respir J 2018; 2018: 9270329. doi: 10.1155/2018/9270329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koo BB, Patel SR, Strohl K, et al. . Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest 2008; 134: 1156–1161. doi: 10.1378/chest.08-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koo BB, Dostal J, Ioachimescu O, et al. . The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath 2008; 12: 259–264. doi: 10.1007/s11325-007-0161-7 [DOI] [PubMed] [Google Scholar]
- 57.Conwell W, Patel B, Doeing D, et al. . Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath 2012; 16: 519–526. doi: 10.1007/s11325-011-0537-6 [DOI] [PubMed] [Google Scholar]
- 58.Mano M, Hoshino T, Sasanabe R, et al. . Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health 2019; 16: 1068. doi: 10.3390/ijerph16061068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cistulli PA, Barnes DJ, Grunstein RR, et al. . Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax 1994; 49: 699–702. doi: 10.1136/thx.49.7.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rimpila V, Lampio L, Kalleinen N, et al. . Evolution of sleep-disordered breathing and blood pressure during menopausal transition. J Sleep Res 2023; 32: e13829. doi: 10.1111/jsr.13829 [DOI] [PubMed] [Google Scholar]
- 61.Huang W, Zhang X, Wang X, et al. . Effects of obstructive sleep apnea during rapid eye movement sleep on cardiac autonomic dysfunction: results from the Shanghai sleep health study cohort. J Sleep Res 2023; 32: e13904. doi: 10.1111/jsr.13904 [DOI] [PubMed] [Google Scholar]
- 62.Somers VK, Dyken ME, Clary MP, et al. . Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96: 1897–1904. doi: 10.1172/JCI118235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donadio V, Liguori R, Vetrugno R, et al. . Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. J Sleep Res 2007; 16: 327–332. doi: 10.1111/j.1365-2869.2007.00602.x [DOI] [PubMed] [Google Scholar]
- 64.Lombardi C, Parati G, Cortelli P, et al. . Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res 2008; 17: 263–270. doi: 10.1111/j.1365-2869.2008.00659.x [DOI] [PubMed] [Google Scholar]
- 65.Chen B, Somers VK, Sun Q, et al. . Implications of sympathetic activation for objective versus self-reported daytime sleepiness in obstructive sleep apnea. Sleep 2022; 45: zsac076. [DOI] [PubMed] [Google Scholar]
- 66.Hoshino T, Sasanabe R, Mano M, et al. . Prevalence of rapid eye movement-related obstructive sleep apnea in adult narcolepsy. Intern Med 2019; 58: 2151–2157. doi: 10.2169/internalmedicine.2601-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung YJ, Oh E. Is REM sleep behavior disorder a friend or foe of obstructive sleep apnea? Clinical and etiological implications for neurodegeneration. J Clin Sleep Med 2021; 17: 1305–1312. doi: 10.5664/jcsm.9144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jo S, Kim HW, Jeon JY, et al. . Protective effects of REM sleep without atonia against obstructive sleep apnea in patients with idiopathic REM sleep behavior disorder. Sleep Med 2019; 54: 116–120. doi: 10.1016/j.sleep.2018.10.032 [DOI] [PubMed] [Google Scholar]
- 69.Giardino DL, Fasano P, Garay A. The “respiratory REM sleep without atonia benefit” on coexisting REM sleep behavior disorder – obstructive sleep apnea. Sleep Sci 2021; 14: 181–185. doi: 10.5935/1984-0063.20200054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee WJ, Sunwoo JS, Byun JI, et al. . Isolated rapid eye movement sleep behavior disorder combined with obstructive sleep apnea: response to treatment and its associated factors. Sleep Med 2022; 91: 75–83. doi: 10.1016/j.sleep.2021.11.021 [DOI] [PubMed] [Google Scholar]
- 71.Berson SR, Klimczak JA, Prezio EA, et al. . House dust mite related allergic rhinitis and REM sleep disturbances. Am J Otolaryngol 2020; 41: 102709. doi: 10.1016/j.amjoto.2020.102709 [DOI] [PubMed] [Google Scholar]
- 72.Novakova SM, Staevska MT, Novakova PI, et al. . Quality of life improvement after a three-year course of sublingual immunotherapy in patients with house dust mite and grass pollen induced allergic rhinitis: results from real-life. Health Qual Life Outcomes 2017; 15: 189. doi: 10.1186/s12955-017-0764-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balcan B, Celik Y, Newitt J, et al. . REM-predominant obstructive sleep apnea in patients with coronary artery disease. J Clin Med 2022; 11: 4402. doi: 10.3390/jcm11154402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aung AT, Kristanto W, Tan MJ, et al. . Obstructive sleep apnea during rapid eye movement sleep in patients after percutaneous coronary intervention: a multicenter study. Sleep Breath 2021; 25: 125–133. doi: 10.1007/s11325-020-02057-6 [DOI] [PubMed] [Google Scholar]
- 75.Punjabi NM, Patil S, Crainiceanu C, et al. . Variability and misclassification of sleep apnea severity based on multi-night testing. Chest 2020; 158: 365–373. doi: 10.1016/j.chest.2020.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lechat B, Naik G, Reynolds A, et al. . Multinight prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med 2022; 205: 563–569. doi: 10.1164/rccm.202107-1761OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chervin RD, Aldrich MS. The relation between multiple sleep latency test findings and the frequency of apneic events in REM and non-REM sleep. Chest 1998; 113: 980–984. doi: 10.1378/chest.113.4.980 [DOI] [PubMed] [Google Scholar]
- 78.Kingshott RN, Engleman HM, Deary IJ, et al. . Does arousal frequency predict daytime function? Eur Respir J 1998; 12: 1264–1270. doi: 10.1183/09031936.98.12061264 [DOI] [PubMed] [Google Scholar]
- 79.Gabryelska A, Bialasiewicz P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci Rep 2020; 10: 34. doi: 10.1038/s41598-019-56478-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Punjabi NM, Bandeen-Roche K, Marx JJ, et al. . The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep 2002; 25: 307–314. doi: 10.1038/s41598-019-56478-9 [DOI] [PubMed] [Google Scholar]
- 81.Varga AW, Kishi A, Mantua J, et al. . Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J Neurosci 2014; 34: 14571–14577. doi: 10.1523/JNEUROSCI.3220-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan KC, Au CT, Yu MW, et al. . Natural history of REM-OSA in children and its associations with adverse blood pressure outcomes: a longitudinal follow-up study. Nat Sci Sleep 2021; 13: 1967–1984. doi: 10.2147/NSS.S331389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoshino T, Sasanabe R, Murotani K, et al. . Insomnia as a symptom of rapid eye movement-related obstructive sleep apnea. J Clin Med 2020; 9: 1821. doi: 10.3390/jcm9061821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin 2022; 17: 173–191. doi: 10.1016/j.jsmc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 85.Djonlagic I, Guo M, Igue M, et al. . REM-related obstructive sleep apnea: when does it matter? Effect on motor memory consolidation versus emotional health. J Clin Sleep Med 2020; 16: 377–384. doi: 10.5664/jcsm.8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, Su C, Liu R, et al. . NREM-AHI greater than REM-AHI versus REM-AHI greater than NREM-AHI in patients with obstructive sleep apnea: clinical and polysomnographic features. Sleep Breath 2011; 15: 463–470. doi: 10.1007/s11325-010-0358-z [DOI] [PubMed] [Google Scholar]
- 87.Mokhlesi B, Hagen EW, Finn LA, et al. . Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 2015; 70: 1062–1069. doi: 10.1136/thoraxjnl-2015-207231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Falla C, Young A, Pope A, et al. . Obstructive sleep apnea during REM sleep: effects on morning and evening blood pressure. Sleep 2023; 46: zsac259. doi: 10.1093/sleep/zsac259 [DOI] [PubMed] [Google Scholar]
- 89.Ren R, Zhang Y, Yang L, et al. . Sleep fragmentation during rapid eye movement sleep and hypertension in obstructive sleep apnea. J Hypertens 2023; 41: 310–315. doi: 10.1097/HJH.0000000000003332 [DOI] [PubMed] [Google Scholar]
- 90.Su W, Chen G, Ma D, et al. . Higher apnea-hypopnea index (AHI) and oxygen desaturation index (ODI) were independently associated with increased risks of hypertension in patients with T2DM: a cross-sectional study. Int J Hypertens 2021; 2021: 8887944. doi: 10.1155/2021/8887944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patil SP, Ayappa IA, Caples SM, et al. . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2019; 15: 335–343. doi: 10.5664/jcsm.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zinchuk AV, Jeon S, Koo BB, et al. . Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax 2018; 73: 472–480. doi: 10.1136/thoraxjnl-2017-210431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pamidi S, Wroblewski K, Broussard J, et al. . Obstructive sleep apnea in young lean men: impact on insulin sensitivity and secretion. Diabetes Care 2012; 35: 2384–2389. doi: 10.2337/dc12-0841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin QC, Zhang XB, Chen GP, et al. . Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath 2012; 16: 571–578. doi: 10.1007/s11325-011-0544-7 [DOI] [PubMed] [Google Scholar]
- 95.Shpirer I, Rapoport MJ, Stav D, et al. . Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath 2012; 16: 461–466. doi: 10.1007/s11325-011-0525-x [DOI] [PubMed] [Google Scholar]
- 96.Bialasiewicz P, Czupryniak L, Pawlowski M, et al. . Sleep disordered breathing in REM sleep reverses the downward trend in glucose concentration. Sleep Med 2011; 12: 76–82. doi: 10.1016/j.sleep.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 97.Grimaldi D, Beccuti G, Touma C, et al. . Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care 2014; 37: 355–363. doi: 10.2337/dc13-0933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chami HA, Gottlieb DJ, Redline S, et al. . Association between glucose metabolism and sleep-disordered breathing during REM sleep. Am J Respir Crit Care Med 2015; 192: 1118–1126. doi: 10.1164/rccm.201501-0046OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koo DL, Kim HR, Nam H. Moderate to severe obstructive sleep apnea during REM sleep as a predictor of metabolic syndrome in a Korean population. Sleep Breath 2020; 24: 1751–1758. doi: 10.1007/s11325-019-02005-z [DOI] [PubMed] [Google Scholar]
- 100.McCullough LM, Mokhlesi B. Obstructive sleep apnea during REM sleep and hypertension: new findings from a clinical cohort. Sleep 2023; 46: zsad003. doi: 10.1093/sleep/zsad003 [DOI] [PubMed] [Google Scholar]
- 101.Gerves-Pinquie C, Bailly S, Goupil F, et al. . Positive airway pressure adherence, mortality, and cardiovascular events in patients with sleep apnea. Am J Respir Crit Care Med 2022; 206: 1393–1404. doi: 10.1164/rccm.202202-0366OC [DOI] [PubMed] [Google Scholar]
- 102.Su CS, Liu KT, Panjapornpon K, et al. . Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med 2012; 8: 243–247. doi: 10.5664/jcsm.1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Almeneessier AS, Almousa Y, Hammad O, et al. . Long-term adherence to continuous positive airway pressure in patients with rapid eye movement-only obstructive sleep apnea: a prospective cohort study. J Thorac Dis 2017; 9: 3755–3765. doi: 10.21037/jtd.2017.09.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoshino T, Sasanabe R, Tanigawa T, et al. . Effect of rapid eye movement-related obstructive sleep apnea on adherence to continuous positive airway pressure. J Int Med Res 2018; 46: 2238–2248. doi: 10.1177/0300060518758583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu XY, Cho JG, Perri R, et al. . CPAP treatment in REM-related obstructive sleep apnea: a distinct clinical phenotype of sleep disordered breathing. Sleep Breath 2021; 25: 1875–1884. doi: 10.1007/s11325-021-02300-8 [DOI] [PubMed] [Google Scholar]
- 106.Ganguly G. The clinical dilema: to treat or not to treat REM related obstructive sleep apnea? Sleep 2012; 35: 755. doi: 10.5665/sleep.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishio Y, Hoshino T, Murotani K, et al. . Treatment outcome of oral appliance in patients with REM-related obstructive sleep apnea. Sleep Breath 2020; 24: 1339–1347. doi: 10.1007/s11325-019-01966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wasey W, Manahil N, Wasey N, et al. . Intraoral neuromuscular stimulation device and rapid eye movement-dependent obstructive sleep apnea. Cureus 2022; 14: e27418. doi: 10.7759/cureus.27418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bughin F, Desplan M, Mestejanot C, et al. . Effects of an individualized exercise training program on severity markers of obstructive sleep apnea syndrome: a randomised controlled trial. Sleep Med 2020; 70: 33–42. doi: 10.1016/j.sleep.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 110.Shechter A, Foster GD, Lang W, et al. . Effects of a lifestyle intervention on REM sleep-related OSA severity in obese individuals with type 2 diabetes. J Sleep Res 2017; 26: 747–755. doi: 10.1111/jsr.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bartolucci ML, Berteotti C, Alvente S, et al. . Obstructive sleep apneas naturally occur in mice during REM sleep and are highly prevalent in a mouse model of Down syndrome. Neurobiol Dis 2021; 159: 105508. doi: 10.1016/j.nbd.2021.105508 [DOI] [PubMed] [Google Scholar]
- 112.Zong S, Du P, Li H, et al. . Advances in animal models of obstructive sleep apnea. Front Med 2023; 10: 988752. doi: 10.3389/fmed.2023.988752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polotsky VY, Rubin AE, Balbir A, et al. . Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 2006; 7: 7–16. doi: 10.1016/j.sleep.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 114.Hendricks JC, Kline LR, Kovalski RJ, et al. . The English bulldog: a natural model of sleep-disordered breathing. J Appl Physiol 1987; 63: 1344–1350. doi: 10.1152/jappl.1987.63.4.1344 [DOI] [PubMed] [Google Scholar]
- 115.Duce B, Kulkas A, Langton C, et al. . The prevalence of REM-related obstructive sleep apnoea is reduced by the AASM 2012 hypopnoea criteria. Sleep Breath 2018; 22: 57–64. doi: 10.1007/s11325-017-1526-1 [DOI] [PubMed] [Google Scholar]
- 116.Gillman A, Roebuck T, Ho S, et al. . Comparison of supine-only and REM-only obstructive sleep apnoea. Sleep Med 2012; 13: 875–878. doi: 10.1016/j.sleep.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 117.Oksenberg A, Arons E, Nasser K, et al. . REM-related obstructive sleep apnea: the effect of body position. J Clin Sleep Med 2010; 6: 343–348. doi: 10.5664/jcsm.27875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pevernagie DA, Gnidovec-Strazisar B, Grote L, et al. . On the rise and fall of the apnea–hypopnea index: a historical review and critical appraisal. J Sleep Res 2020; 29: e13066. doi: 10.1111/jsr.13066 [DOI] [PubMed] [Google Scholar]
- 119.Muraja-Murro A, Kulkas A, Hiltunen M, et al. . Adjustment of apnea–hypopnea index with severity of obstruction events enhances detection of sleep apnea patients with the highest risk of severe health consequences. Sleep Breath 2014; 18: 641–647. doi: 10.1007/s11325-013-0927-z [DOI] [PubMed] [Google Scholar]
- 120.Azarbarzin A, Sands SA, White DP, et al. . The hypoxic burden: a novel sleep apnoea severity metric and a predictor of cardiovascular mortality–Reply to “The hypoxic burden: also known as the desaturation severity parameter”. Eur Heart J 2019; 40: 2994–2995. doi: 10.1093/eurheartj/ehz273 [DOI] [PubMed] [Google Scholar]