Abstract
Background
A pneumoperitoneum of 12 to 16 mm Hg is used for laparoscopic cholecystectomy. Lower pressures are claimed to be safe and effective in decreasing cardiopulmonary complications and pain.
Objectives
To assess the benefits and harms of low pressure pneumoperitoneum compared with standard pressure pneumoperitoneum in people undergoing laparoscopic cholecystectomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until February 2013 to identify randomised trials,
using search strategies.
Selection criteria
We considered only randomised clinical trials, irrespective of language, blinding, or publication status for inclusion in the review.
Data collection and analysis
Two review authors independently identified trials and independently extracted data. We calculated the risk ratio (RR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals (CI) using both fixed‐effect and random‐effects models with RevMan 5 based on available case analysis.
Main results
A total of 1092 participants randomly assigned to the low pressure group (509 participants) and the standard pressure group (583 participants) in 21 trials provided information for this review on one or more outcomes. Three additional trials comparing low pressure pneumoperitoneum with standard pressure pneumoperitoneum (including 179 participants) provided no information for this review. Most of the trials included low anaesthetic risk participants undergoing elective laparoscopic cholecystectomy. One trial including 140 participants was at low risk of bias. The remaining 20 trials were at high risk of bias. The overall quality of evidence was low or very low. No mortality was reported in either the low pressure group (0/199; 0%) or the standard pressure group (0/235; 0%) in eight trials that reported mortality. One participant experienced the outcome of serious adverse events (low pressure group 1/179, 0.6%; standard pressure group 0/215, 0%; seven trials; 394 participants; RR 3.00; 95% CI 0.14 to 65.90; very low quality evidence). Quality of life, return to normal activity, and return to work were not reported in any of the trials. The difference between groups in the conversion to open cholecystectomy was imprecise (low pressure group 2/269, adjusted proportion 0.8%; standard pressure group 2/287, 0.7%; 10 trials; 556 participants; RR 1.18; 95% CI 0.29 to 4.72; very low quality evidence) and was compatible with an increase, a decrease, or no difference in the proportion of conversion to open cholecystectomy due to low pressure pneumoperitoneum. No difference in the length of hospital stay was reported between the groups (five trials; 415 participants; MD ‐0.30 days; 95% CI ‐0.63 to 0.02; low quality evidence). Operating time was about two minutes longer in the low pressure group than in the standard pressure group (19 trials; 990 participants; MD 1.51 minutes; 95% CI 0.07 to 2.94; very low quality evidence).
Authors' conclusions
Laparoscopic cholecystectomy can be completed successfully using low pressure in approximately 90% of people undergoing laparoscopic cholecystectomy. However, no evidence is currently available to support the use of low pressure pneumoperitoneum in low anaesthetic risk patients undergoing elective laparoscopic cholecystectomy. The safety of low pressure pneumoperitoneum has to be established. Further well‐designed trials are necessary, particularly in people with cardiopulmonary disorders who undergo laparoscopic cholecystectomy.
Keywords: Humans; Carbon Dioxide; Cholecystectomy, Laparoscopic; Cholecystectomy, Laparoscopic/methods; Conversion to Open Surgery; Length of Stay; Pneumoperitoneum, Artificial; Pneumoperitoneum, Artificial/adverse effects; Pneumoperitoneum, Artificial/methods; Pressure; Pressure/adverse effects; Randomized Controlled Trials as Topic
Plain language summary
Low pressure pneumoperitoneum versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy
Background
The liver produces bile, which has many functions, including elimination of waste processed by the liver and digestion of fat. Bile is temporarily stored in the gallbladder (an organ situated underneath the liver) before it reaches the small bowel. Concretions in the gallbladder are called gallstones. Gallstones are present in about 5% to 25% of the adult Western population. Between 2% and 4% become symptomatic within a year. Symptoms include pain related to the gallbladder (biliary colic), inflammation of the gallbladder (cholecystitis), obstruction to the flow of bile from the liver and gallbladder into the small bowel resulting in jaundice (yellowish discolouration of the body usually most prominently noticed in the white of the eye, which turns yellow), bile infection (cholangitis), and inflammation of the pancreas, an organ that secretes digestive juices and harbours the insulin‐secreting cells that maintain blood sugar level (pancreatitis). Removal of the gallbladder (cholecystectomy) is currently considered the best treatment option for patients with symptomatic gallstones. This is generally performed by key‐hole surgery (laparoscopic cholecystectomy). Laparoscopic cholecystectomy is generally performed by inflating the tummy with carbon dioxide gas to permit the organs and structures within the tummy to be viewed so that the surgery can be performed. The gas pressure used to inflate the tummy is usually 12 mm Hg to 16 mm Hg (standard pressure). However, this causes alterations in the blood circulation and may be detrimental. To overcome this, lower pressure has been suggested as an alternative to standard pressure. However, using lower pressure may limit the surgeon's view of the organs and structures within the tummy, possibly resulting in inadvertent damage to the organs or structures. The review authors set out to determine whether it is preferable to perform laparoscopic cholecystectomy using low pressure or standard pressure. A systematic search of medical literature was performed to identify studies that provided information on the above question. The review authors obtained information from randomised trials only because such types of trials provide the best information if conducted well. Two review authors independently identified the trials and collected the information.
Study characteristics
A total of 1092 patients were studied in 21 trials. Patients were assigned to a low pressure group (509 patients) or a standard pressure group (583 patients). The choice of treatment was determined by a method similar to the toss of a coin. Most of the trials included low surgical risk patients undergoing planned laparoscopic cholecystectomy.
Key results
Laparoscopic cholecystectomy could be completed successfully using low pressure in approximately 90% of people undergoing this procedure. No deaths were reported in either low pressure or standard pressure groups in eight trials that reported deaths (total of 434 patients in both groups). Seven trials with 394 patients described complications related to surgery. One participant experienced the outcome of serious adverse events (low pressure group 1/179, 0.6%; standard pressure group 0/215, 0%). Quality of life, return to normal activity, and return to work were not reported in any of the trials. The difference in the percentage of people undergoing conversion to open operation (from key‐hole operation) between the low pressure group (2/269; 0.8%) and the standard pressure group (2/287; 0.7%) was imprecise. This was reported in 10 studies. No difference was noted in the length of hospital stay between the groups. Operating time was about two minutes longer (very low quality evidence) in the low pressure group than in the standard pressure group. Currently no evidence is available to support the use of low pressure pneumoperitoneum in low surgical risk patients undergoing planned laparoscopic cholecystectomy. The safety of low pressure pneumoperitoneum has to be established.
Quality of evidence
Only one trial including 140 participants was at low risk of bias (low chance of arriving at wrong conclusions because of study design). The remaining 20 trials were at high risk of bias (high chance of arriving at wrong conclusions because of trial design). The overall quality of evidence was very low.
Future research
Further well‐designed trials are necessary, particularly in high surgical risk patients undergoing laparoscopic cholecystectomy.
Summary of findings
Summary of findings for the main comparison. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy.
| Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy | |||||
| Patient or population: patients undergoing laparoscopic cholecystectomy. Settings: secondary or tertiary. Intervention: low pressure pneumoperitoneum. Comparison: standard pressure pneumoperitoneum. | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Intervention | ||||
| Mortality | No mortality in either group | not estimable | 434 (8 studies) |
⊕⊝⊝⊝ very low1,2 | |
| Serious adverse events | 3 per 1000 | 8 per 1000 (0 to 167) | RR 3 (0.14 to 65.9) | 394 (7 studies) | ⊕⊝⊝⊝ very low1,2 |
| Conversion to open cholecystectomy | 7 per 1000 | 8 per 1000 (2 to 33) | RR 1.18 (0.29 to 4.72) | 556 (10 studies) | ⊕⊝⊝⊝ very low1,2 |
| Hospital stay | The mean hospital stay in the control groups was 2 days | The mean hospital stay in the intervention groups was 0.3 lower (0.63 lower to 0.02 higher) | 415 (5 studies) | ⊕⊝⊝⊝ very low1,3 | |
| Operating time | The mean operating time in the control groups was 55 minutes | The mean operating time in the intervention groups was 1.51 higher (0.07 to 2.94 higher) | 990 (19 studies) | ⊕⊕⊝⊝ low1 | |
| *The basis for the assumed risk is the mean control group risk for conversion to open cholecystectomy. Although we planned to use the mean control group risk for serious adverse events also, we could not do so because no serious adverse events were reported in the control group. Overall serious adverse events in both groups were used as the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1The trial(s) was (were) at high risk of bias (two points). 2The confidence intervals overlapped 1 and either 0.75 or 1.25 or both. Events in the intervention and control groups were fewer than 300 (two points). 3Severe heterogeneity was noted by the I2 and the lack of overlap of confidence intervals (two points).
Background
Description of the condition
About 5% to 25% of the adult Western population have gallstones (GREPCO 1984; GREPCO 1988; Bates 1992; Halldestam 2004). The annual incidence of gallstones is about one in 200 people (NIH 1992). Only 2% to 4% of people with gallstones become symptomatic with biliary colic (pain), acute cholecystitis (inflammation), obstructive jaundice, or gallstone pancreatitis within a year (Attili 1995; Halldestam 2004). Cholecystectomy (removal of the gallbladder) is the preferred option in the treatment of symptomatic gallstones (Strasberg 1993). Every year, more than 0.5 million cholecystectomies are performed in the US and 60,000 in the UK (Dolan 2009; HES 2011). Approximately 80% of cholecystectomies are performed laparoscopically (by key‐hole surgery) (Ballal 2009). Biliary colic (pain in the right upper abdomen lasting longer than half an hour) is one of the symptoms related to gallstones (Berger 2000) and is the most common indication for cholecystectomy (Glasgow 2000).
Description of the intervention
Traditionally, one of the first steps in laparoscopic cholecystectomy is the creation of pneumoperitoneum (Russell 1993) using carbon dioxide (CO2) through a Veress needle (Casati 1997) or through a port (hole) (Alijani 2004) in the abdominal wall. Traditionally, the pressure used is around 15 mm Hg (Russell 1993). The created pneumoperitoneum allows visualisation and manipulation of instruments inside the abdominal cavity. Increased intra‐abdominal pressure due to the pneumoperitoneum causes several cardiopulmonary changes. The increased intra‐abdominal pressure increases the absorption of CO2, causing hypercapnia and acidosis, which must be avoided by hyperventilation (Henny 2005). It also pushes the diaphragm upwards, decreasing pulmonary compliance (Alijani 2004; Henny 2005), and increases the peak airway pressure (Galizia 2001; Alijani 2004). Increased intra‐abdominal pressure increases the venous return due to blood compressed out of the splanchnic vasculature (Henny 2005). Pneumoperitoneum also increases systemic vascular resistance (Galizia 2001; Mertens 2004) and pulmonary vascular resistance (Galizia 2001). Carbon dioxide pneumoperitoneum predisposes to cardiac arrhythmias (Egawa 2006). During the early phase of pneumoperitoneum, cardiac output is reduced (Galizia 2001; Alijani 2004) by decreasing venous return (Neudecker 2002). Although these cardiorespiratory changes may be tolerated by healthy adults with adequate cardiopulmonary reserve, people with cardiopulmonary diseases may not tolerate these cardiopulmonary changes. About 17% of patients undergoing laparoscopic cholecystectomy have an American Society of Anesthesiologists (ASA) status of III or IV (Giger 2006; ASA 2007). Abdominal wall lift, using a special device (eg, Laparolift (Egawa 2006), Laparo‐tensor (Alijani 2004)) introduced through a port in the abdominal wall, has been used to decrease the cardiopulmonary changes and has been considered in a different review (Gurusamy 2012). Helium insufflation is an alternative to CO2 insufflation (Neuhaus 2001) and has been reported to have little or no effect on pulmonary function in pigs (Junghans 1997). However, concerns about the solubility of helium in the blood and hence the risk of gas embolism have precluded its routine use in humans (Neuhaus 2001).
How the intervention might work
Lower pressure may decrease the effects of pneumoperitoneum. However, the safety of low pressure pneumoperitoneum has not been established.
Why it is important to do this review
In our previous version of the review, we found evidence from trials with high risk of bias showing that low pressure pneumoperitoneum decreased pain scores (Gurusamy 2009). However, pain scores are unvalidated surrogate outcomes for pain in people undergoing laparoscopic cholecystectomy, and several Cochrane systematic reviews have demonstrated that pain scores can be decreased with no clinical implications in people undergoing laparoscopic cholecystectomy (Gurusamy 2014a; Gurusamy 2014b; Gurusamy 2014c). In addition, no study has evaluated the level of pain scores that people undergoing laparoscopic cholecystectomy or any other elective or emergency operation consider as important. The minimal clinically important difference in pain scores has also not been established in people undergoing laparoscopic cholecystectomy or any other elective or emergency operation. This update of our previous review includes results from trials that became available since the time of our last review.
Objectives
To assess the benefits and harms of low pressure pneumoperitoneum compared with standard pressure pneumoperitoneum in patients undergoing laparoscopic cholecystectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised clinical trials that compared different pressures of pneumoperitoneum in participants undergoing laparoscopic cholecystectomy (irrespective of language, blinding, publication status, or sample size, or whether the trials were adequately powered). We did not consider randomised trials that compared abdominal wall lift in combination with pneumoperitoneum versus pneumoperitoneum alone. Such trials were included in the review in which abdominal lift and pneumoperitoneum were compared (Gurusamy 2012).
We excluded quasi‐randomised trials (ie, trials in which the method of allocating participants to a treatment were not strictly random, for example, date of birth, hospital record number, or alternation).
Types of participants
Patients undergoing laparoscopic cholecystectomy (elective or emergency) for any reason (symptomatic gallstones, acalculous cholecystitis, gallbladder polyp, or any other condition) using four ports, at least two of 10 mm or larger and the remaining two of 5 mm or larger (which is generally considered as standard laparoscopic cholecystectomy). We excluded trials in which fewer ports or smaller ports were used, as the safety of such procedures has not been established (Gurusamy 2013; Gurusamy 2014d). We applied no restriction based on the type of anaesthesia used provided that the same type of anaesthesia was used in both groups.
Types of interventions
Trials comparing low pressure (less than 12 mm Hg) versus standard pressure (12 to 16 mm Hg) pneumoperitoneum. We excluded any trials using pressure greater than 16 mm Hg. The definitions of standard (12 mm Hg to 16 mm Hg) and low (less than 12 mm Hg) were chosen arbitrarily and were based on general belief and the review authors' opinions. No universal definitions are available for standard and low.
Types of outcome measures
Primary outcomes
Mortality (30‐day or in‐hospital mortality).
Serious adverse events: defined as any events that would increase mortality; are life‐threatening; require inpatient hospitalisation; or result in persistent or significant disability; or any important medical events that might have jeopardised the participant or required intervention for prevention. All other adverse events were considered non‐serious (ICH‐GCP 1997). Combining outcomes of different severity can result in wrong conclusions about the safety and effectiveness of an intervention (Cordoba 2010), and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) recommends that an exhaustive list of all outcomes should not be included—only important outcomes that are important to patients or health policy‐makers; therefore we included only serious adverse events rather than all adverse events.
Quality of life.
Secondary outcomes
Conversion to open cholecystectomy.
-
Hospital stay.
Proportion discharged as day procedure.
Length of hospital stay.
Return to normal activity.
Return to work.
Operating time.
We also collected information on the successful completion of low pressure laparoscopic cholecystectomy.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003). We have provided the search strategies in Appendix 1 along with the time span for the searches. Searches were conducted until February 2013.
Searching other resources
We also searched the references of identified trials to identify further relevant trials.
Data collection and analysis
Selection of studies
KSG and JV independently identified the trials for inclusion. We have listed the excluded studies along with the reasons for exclusion.
Data extraction and management
KSG and JV independently extracted the following data.
Year and language of publication.
Country.
Year of study.
Inclusion and exclusion criteria.
Sample size.
Population characteristics such as age and sex ratio.
Details of intervention and control.
Co‐interventions.
Outcomes (listed above).
Risk of bias (described below).
Assessment of risk of bias in included studies
We independently assessed the risk of bias in the trials without masking the trial names. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012). Based on the risk of biased overestimation of beneficial intervention effects in randomised trials with high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savović 2012; Savović 2012a), we assessed the trials for the following risk of bias domains.
Allocation sequence generation
Low risk of bias: Sequence generation was achieved using a computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: The method of sequence generation was not specified.
High risk of bias: The sequence generation method was not random.
Allocation concealment
Low risk of bias: The participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (eg, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: The method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: The allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants, personnel, and outcome assessors
Low risk of bias: Blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: Information was insufficient to allow assessment of whether blinding was likely to induce bias on the results.
High risk of bias: No blinding or incomplete blinding was provided, and assessment of outcomes was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: Missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: Information was insufficient to allow assessment of whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: The results were likely to be biased because of missing data.
Selective outcome reporting
Low risk of bias: All outcomes were predefined and reported, or all clinically relevant and reasonably expected outcomes were reported.
Uncertain risk of bias: It is unclear whether all predefined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: One or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
For‐profit bias
Low risk of bias: The trial appears to be free of industry sponsorship or other kinds of for‐profit support that may lead to manipulatiion of trial design, conductance, or results.
Uncertain risk of bias: The trial may or may not be free of for‐profit bias, as no information on clinical trial support or sponsorship is provided.
High risk of bias: The trial is sponsored by the industry or has received other kinds of for‐profit support.
We considered trials to have a low risk of bias if we assessed all of the above domains as being at low risk of bias. In all other cases, the trials were considered to have a high risk of bias.
Measures of treatment effect
For binary outcomes, we calculated the risk ratio (RR) with 95% confidence interval (CI). We also planned to report the risk difference if the conclusions would have changed by using risk difference, because risk difference allows meta‐analysis including trials with zero events in both groups. For continuous variables, we calculated the mean difference (MD) with 95% CI for hospital stay as well as standardised mean difference (SMD) with 95% CI for variables such as quality of life.
Unit of analysis issues
The unit of analysis was the participant undergoing laparoscopic cholecystectomy.
Dealing with missing data
We performed an intention‐to‐treat analysis (Newell 1992) when possible for binary outcomes. For continuous outcomes, we used available‐case analysis in the presence of missing data unless the study authors reported an intention‐to‐treat analysis based on an appropriate method of imputation of data such as multiple imputation. We planned to use intention‐to‐treat analysis if such analysis was available. We imputed the standard deviation from P values according to instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CIs, we imputed the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation would decrease the weight of the trial for calculation of mean differences and would bias the effect estimate to no effect in the case of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We examined the forest plot to visually assess heterogeneity. We used overlapping of CIs to visually assess heterogeneity. We explored heterogeneity by using the Chi2 test, with significance set at a P value of 0.10, and measured the quantity of heterogeneity using the I2 statistic (Higgins 2002).
Assessment of reporting biases
We used a funnel plot to explore bias in the presence of at least 10 trials for the outcome (Egger 1997; Macaskill 2001). We used asymmetry in the funnel plot of trial size against treatment effect to assess this bias. We also used the linear regression approach described by Egger et al to determine the funnel plot asymmetry (Egger 1997).
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012), using the software package Review Manager 5 (RevMan 2012). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In the case of a discrepancy between the two models, we have reported both results; otherwise, we have reported only the results from the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Trials with low risk of bias versus trials with high risk of bias.
Different gases used for pneumoperitoneum.
Different pressures used for pneumoperitoneum (borderline low 10 mm Hg to 11 mm Hg; moderately low 7 mm Hg to 9 mmHg; and very low up to 6 mm Hg). This was defined arbitrarily again based on general belief and on review authors' opinions because no universal definitions are available.
Elective versus emergency cholecystectomy.
We planned to perform the Chi2 test for subgroup differences, setting a P value of 0.05 to identify any differences for the subgroup analyses.
Sensitivity analysis
We planned to perform a sensitivity analysis by excluding the trials in which medians or standard deviations were imputed for continuous outcomes.
Trial sequential analysis
We planned to use trial sequential analysis to control for random errors due to sparse data and repetitive testing of accumulating data (CTU 2011; Thorlund 2011). The underlying assumption of trial sequential analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis, resulting in an increased risk of random errors. We planned to add the trials according to the year of publication, and if more than one trial was published in a year, we planned to add the trials alphabetically according to the last name of the first author. We planned to construct trial sequential monitoring boundaries on the basis of the required information size. These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size; if the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may perhaps be established and further trials may turn out to be superfluous. On the other hand, if the boundary is not surpassed, it may be necessary to continue doing trials to detect or reject a certain intervention effect (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010).
We planned to apply trial sequential analysis (CTU 2011; Thorlund 2011) using a diversity‐adjusted required information size (DARIS) calculated from an alpha error of 0.05, a beta error of 0.20, a control event proportion obtained from the results, and a relative risk reduction of 20% for binary outcomes with two or more trials to determine whether more trials on this topic are necessary. Trial sequential analysis cannot be performed for standardised mean difference. So, we did not plan to perform a trial sequential analysis for quality of life. For hospital stay, return to normal activity, and return to work, we planned to calculate the DARIS from an alpha error of 0.05, a beta error of 0.20, the variance estimated from the meta‐analysis results of low risk of bias trials (if available), and a minimal clinically relevant difference of one day. For operating time, we planned to calculate the DARIS using a minimal clinically relevant difference of 15 minutes, with remaining parameters the same as for hospital stay.
Summary of findings table
We have summarised the results of all outcomes in a 'Summary of findings' table prepared using GRADEPro 3.6 (http://ims.cochrane.org/revman/gradepro).
Results
Description of studies
Results of the search
We identified a total of 1057 bibliographic references through electronic searches of The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 202), MEDLINE (n = 240), EMBASE (n = 233), and Science Citation Index Expanded (n = 382). We excluded 465 duplicates and 555 clearly irrelevant references through reading abstracts. Thirty‐eight references were retrieved for further assessment. One reference was identified through contacting experts in the field. No references were identified by scanning reference lists of the identified randomised trials. We excluded 12 references of 11 studies for the reasons listed under the table ‘Characteristics of excluded studies’. Twenty‐six references of 24 randomised clinical trials were included in the review. Twenty‐one randomised clinical trials provided data for this review. The reference flow is shown in Figure 1. The details of population characteristics, pressure used for pneumoperitoneum, and outcomes reported by individual trials are shown in the table ‘Characteristics of included studies’.
1.
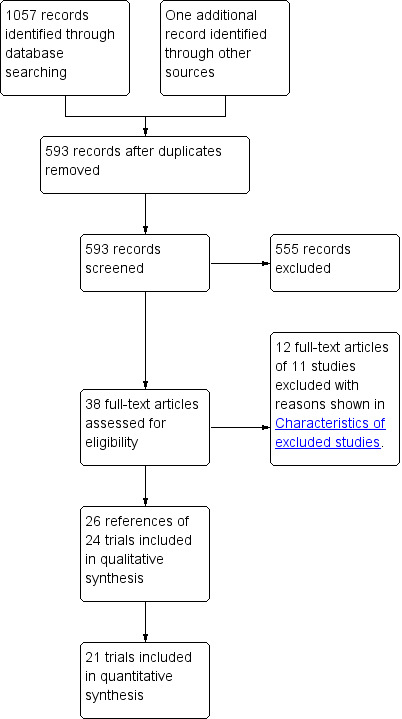
Study flow diagram.
Included studies
A total of 1277 participants were randomly assigned in the 24 trials included in this review (Pier 1994; Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2002; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Kanwer 2009; Sandhu 2009; Torres 2009; Celik 2010; Kandil 2010; Topal 2011; Eryilmaz 2012). However, only 21 trials including 1092 participants provided information for this review and further description about participants and interventions (Pier 1994; Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Kanwer 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011). Participants were randomly assigned to the low pressure group (509 participants) and the standard pressure group (583 participants) in the 21 trials (Pier 1994; Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Kanwer 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011). The average age of participants ranged between 42 years and 58 years in the 19 trials that provided this information (Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011). The proportion of female participants ranged between 21.7% and 100% in the 19 trials that provided this information (Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011). Twenty trials included only participants undergoing elective laparoscopic cholecystectomy (Pier 1994; Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Kanwer 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011). It was not clear whether participants undergoing emergency laparoscopic cholecystectomy were included in one trial (Polat 2003). Eleven trials clearly stated that they included only ASA I or II (low anaesthetic risk) participants (Pier 1994; Dexter 1999; Barczynski 2003; Perrakis 2003; Sefr 2003; Basgul 2004; Hasukic 2005; Chok 2006; Karagulle 2008; Sandhu 2009; Celik 2010). One trial included ASA I to III participants (Koc 2005). This information was not available for the remaining nine trials (Unbehaum 1995; Wallace 1997; Polat 2003; Celik 2004; Ibraheim 2006; Joshipura 2009; Kanwer 2009; Torres 2009; Topal 2011). All 21 trials used carbon dioxide pneumoperitoneum (Pier 1994; Unbehaum 1995; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Kanwer 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011).
Interventions
The types of low pressure used in the different trials were as follows.
Borderline low (10 mm Hg to 11 mm Hg): six trials (Polat 2003; Sefr 2003; Basgul 2004; Koc 2005; Kanwer 2009; Topal 2011).
Moderately low (7 mm Hg to 9 mm Hg): 13 trials (Pier 1994; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Hasukic 2005; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Sandhu 2009; Torres 2009; Celik 2010).
Very low (up to 6 mm Hg): no trials.
In the remaining two trials, the pressure used was 8 to 10 mm Hg (Unbehaum 1995; Celik 2004).
In one trial, the trocar was inserted at standard pressure and the operation was performed under low pressure (Joshipura 2009).
Risk of bias in included studies
The risk of bias of all trials included in the review is shown in Figure 2. The risk of bias in individual trials is shown in Figure 3. Nine trials had low risk of bias in the allocation sequence generation domain (Wallace 1997; Dexter 1999; Barczynski 2003; Sefr 2003; Hasukic 2005; Chok 2006; Kanwer 2009; Sandhu 2009; Celik 2010). Eleven trials had low risk of bias in the allocation concealment domain (Dexter 1999; Barczynski 2002; Barczynski 2003; Perrakis 2003; Sefr 2003; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Joshipura 2009; Sandhu 2009). Three trials had low risk of bias in the blinding of participants, personnel, and outcome assessors domain (Wallace 1997; Joshipura 2009; Sandhu 2009). Ten trials had low risk of bias due to missing outcome data (Wallace 1997; Barczynski 2002; Barczynski 2003; Perrakis 2003; Sefr 2003; Celik 2004; Hasukic 2005; Chok 2006; Joshipura 2009; Sandhu 2009). Seven trials had low risk of bias due to selective outcome reporting (Wallace 1997; Dexter 1999; Hasukic 2005; Chok 2006; Karagulle 2008; Sandhu 2009; Celik 2010). Four trials had low risk of bias in the for‐profit bias domain (Barczynski 2002; Chok 2006; Kanwer 2009; Sandhu 2009). Only one trial was considered to be at low risk of bias (Sandhu 2009).
2.
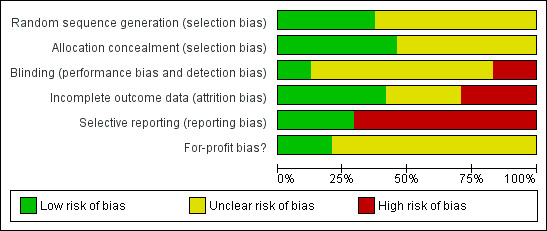
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
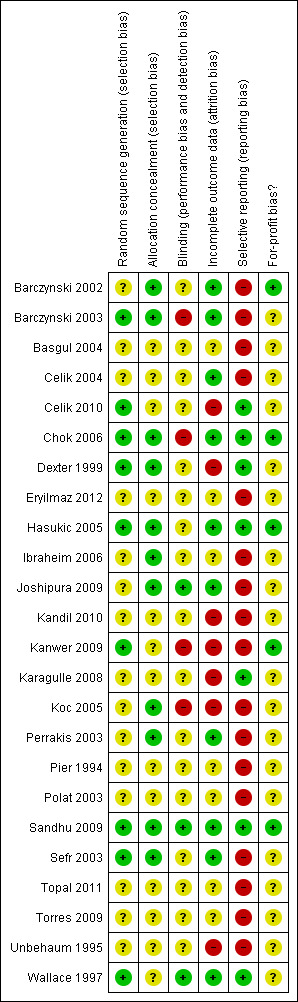
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Table 1
The results are summarised in Table 1.
Mortality
Mortality was reported in eight trials (Wallace 1997; Dexter 1999; Perrakis 2003; Hasukic 2005; Chok 2006; Karagulle 2008; Sandhu 2009; Celik 2010). No mortality was reported in either the low pressure group (0/199; 0%) or the standard pressure group (0/235; 0%). As no mortality was reported in either group, we were unable to use the control group proportion for calculation of the required information size of the trial sequential analysis. Instead, we used a proportion of 0.2% in the control group based on data from approximately 30,000 patients included in a database in Switzerland (Giger 2011). The proportion of information accrued was only 0.12% of the DARIS, and so the trial sequential monitoring boundaries were not drawn (Figure 4). The cumulative Z‐curve does not cross the conventional statistical boundaries.
4.
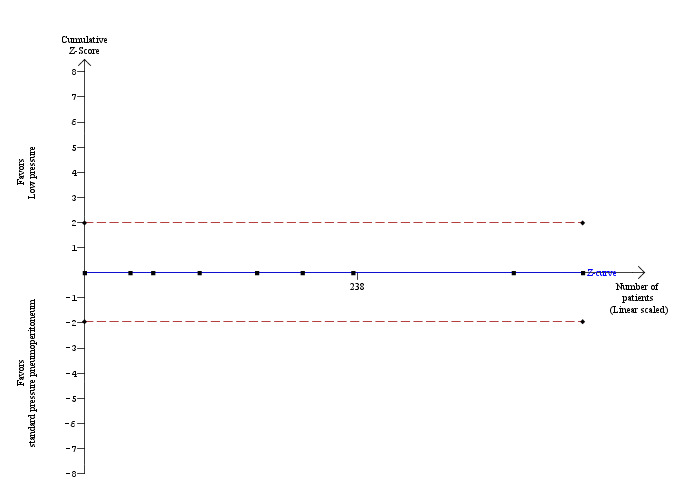
Trial sequential analysis of mortality The diversity‐adjusted required information size (DARIS) was calculated to 352,564 participants, based on the proportion of participants in the control group with the outcome of 0.2%, for a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. After accrual of 434 participants in the eight trials, only 0.12% of the DARIS has been reached. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, not even the conventional boundaries (dotted red line) were crossed by the cumulative Z‐curve.
Serious adverse events
Serious adverse events were reported in seven trials (Wallace 1997; Dexter 1999; Hasukic 2005; Chok 2006; Karagulle 2008; Sandhu 2009; Celik 2010). No significant difference was noted in the proportions of participants with serious adverse events between the low pressure group (1/179; 0.6%) and the standard pressure group (0/215; 0%) (RR 3.00, 95% CI 0.14 to 65.90) (Analysis 1.1). As only serious adverse events were reported in only one trial, the issue of fixed‐effect model versus random‐effects model does not arise. As no serious adverse events were reported in the control group, we used the overall proportions in both groups as the control group proportion for performing trial sequential analysis. The proportion of information accrued was only 0.14% of the DARIS, and so the trial sequential monitoring boundaries were not drawn (Figure 5). The cumulative Z‐curve does not cross the conventional statistical boundaries.
1.1. Analysis.
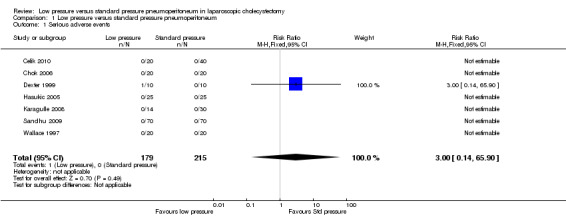
Comparison 1 Low pressure versus standard pressure pneumoperitoneum, Outcome 1 Serious adverse events.
5.
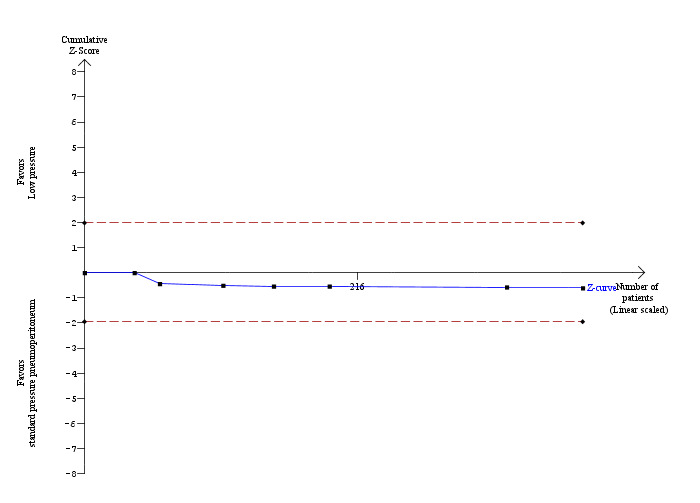
Trial sequential analysis of serious adverse events The diversity‐adjusted required information size (DARIS) was calculated to 281,924 participants, based on the proportion of participants in the control group with the outcome of 0.25%, for a relative risk reduction of 20%, an alpha of 5%, a beta of 20% and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accrual of 394 participants in the seven trials, only 0.14% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, not even the conventional boundaries (dotted red line) were crossed by the cumulative Z‐curve.
Quality of life
Quality of life was not reported in any of the trials.
Conversion to open cholecystectomy
Conversion to open cholecystectomy was reported in 10 trials (Dexter 1999; Barczynski 2003; Perrakis 2003; Sefr 2003; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Sandhu 2009; Celik 2010). No significant difference in the conversion to open cholecystectomy was observed between the low pressure group (2/269; 0.7%) and the standard pressure group (2/287; 0.7%) (RR 1.18, 95% CI 0.29 to 4.72) (Analysis 1.2). The trial sequential analysis revealed that the proportion of information accrued was only 0.55% of the DARIS, and so the trial sequential monitoring boundaries were not drawn (Figure 6). The cumulative Z‐curve does not cross the conventional statistical boundaries.
1.2. Analysis.
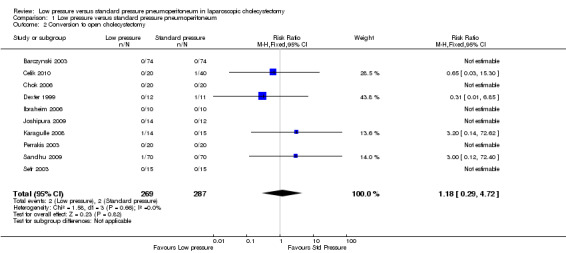
Comparison 1 Low pressure versus standard pressure pneumoperitoneum, Outcome 2 Conversion to open cholecystectomy.
6.
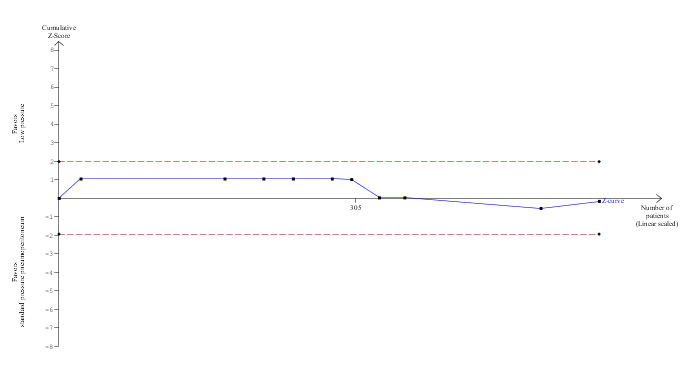
Trial sequential analysis of conversion to open cholecystectomy The diversity‐adjusted required information size (DARIS) was calculated to 100,279 participants, based on the proportion of participants in the control group with the outcome of 0.70%, for a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accrual of 556 participants in the 10 trials, only 0.55% of the DARIS has been reached. Accordingly, the trial sequential analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, not even the conventional boundaries (dotted red line) were crossed by the cumulative Z‐curve.
Hospital stay
None of the trials reported the proportion discharged as day‐procedure laparoscopic cholecystectomy. Length of hospital stay was reported in five trials (Unbehaum 1995; Wallace 1997; Barczynski 2003; Joshipura 2009; Sandhu 2009). Hospital stay was statistically shorter in the low pressure group than in the standard pressure group by the fixed‐effect model (MD ‐0.27 days, 95% CI ‐0.36 to ‐0.17) (Analysis 1.3). This difference was not clinically significant. No significant difference was noted between the groups using the random‐effects model (MD ‐0.30 days, 95% CI ‐0.63 to 0.02). No imputation of mean or standard deviation was performed, and so the sensitivity analysis was not performed. The trial sequential analysis suggested that it is unlikely that future trials will demonstrate any significant difference in length of hospital stay between low pressure groups and standard pressure groups as the cumulative Z‐curve has crossed the DARIS but does not cross the conventional statistical boundaries (Figure 7).
1.3. Analysis.
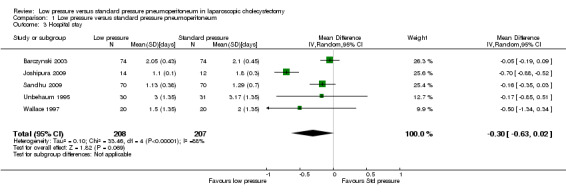
Comparison 1 Low pressure versus standard pressure pneumoperitoneum, Outcome 3 Hospital stay.
7.
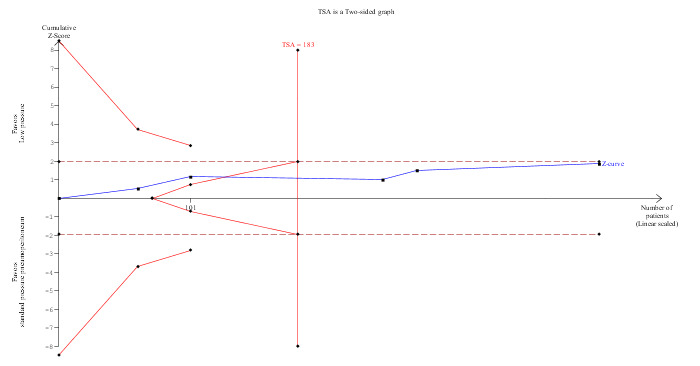
Trial sequential analysis of hospital stay The diversity‐adjusted required information size (DARIS) was 183 participants based on a minimal relevant difference (MIRD) of one day, a variance (VAR) of 0.47, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 91.83%. Neither the conventional statistical boundaries (dotted red line) nor the trial sequential monitoring boundaries (red line) are crossed by the cumulative Z‐curve (blue line), although the DARIS has been reached. The findings are consistent with no significant difference in length of hospital stay between low pressure and standard pressure pneumoperitoneum with low risk of random errors.
Return to normal activity
None of the trials reported this outcome.
Return to work
None of the trials reported this outcome.
Operating time
Operating time was reported in 19 trials (Pier 1994; Wallace 1997; Dexter 1999; Barczynski 2003; Perrakis 2003; Polat 2003; Sefr 2003; Basgul 2004; Celik 2004; Hasukic 2005; Koc 2005; Chok 2006; Ibraheim 2006; Joshipura 2009; Kanwer 2009; Sandhu 2009; Torres 2009; Celik 2010; Topal 2011). Operating time was about two minutes longer in the low pressure group than in the standard pressure group (MD 1.51 minutes, 95% CI 0.07 to 2.94) (Analysis 1.4). No change in results was noted when the random‐effects model was used. The mean or the standard deviation or both were imputed in five trials (Wallace 1997; Dexter 1999; Perrakis 2003; Torres 2009; Celik 2010). Excluding these trials from the analysis did not alter the results. The trial sequential analysis revealed that the DARIS has been crossed. The conventional statistical boundaries were crossed by a cumulative Z‐curve favouring standard pressure pneumoperitoneum. Findings were consistent with low pressure pneumoperitoneum resulting in longer operating time compared with standard pressure pneumoperitoneum with low risk of random errors (Figure 8).
1.4. Analysis.
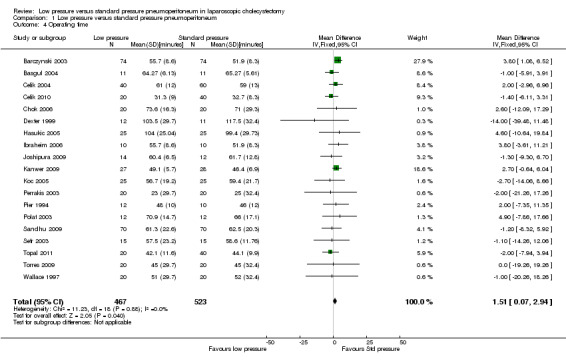
Comparison 1 Low pressure versus standard pressure pneumoperitoneum, Outcome 4 Operating time.
8.
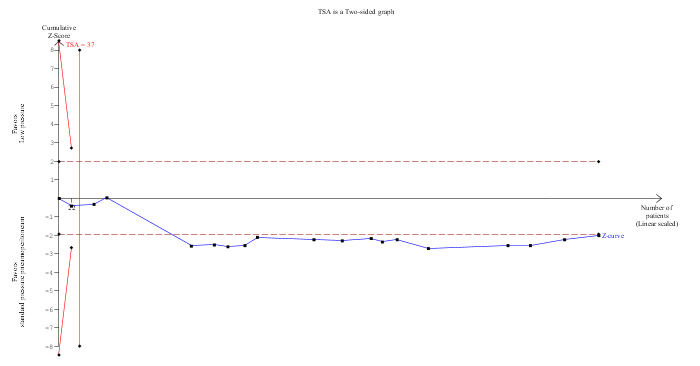
Trial sequential analysis of operating time The diversity‐adjusted required information size (DARIS) was 37 participants based on a minimal relevant difference (MIRD) of 15 minutes, a variance (VAR) of 258.34, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. The conventional statistical boundaries (dotted red line) are crossed by the cumulative Z‐curve (blue line) after the fourth trial. The trial sequential monitoring boundary (red line) is crossed by the cumulative Z‐curve after the second trial. The findings are consistent with low pressure pneumoperitoneum associated with a longer operating time than standard pressure pneumoperitoneum with low risk of random errors.
Successful completion of low pressure laparoscopic cholecystectomy
Successful completion of low pressure laparoscopic cholecystectomy was reported in nine trials (Wallace 1997; Barczynski 2003; Perrakis 2003; Chok 2006; Ibraheim 2006; Joshipura 2009; Kanwer 2009; Sandhu 2009; Celik 2010). The median proportion of successful completion of low pressure laparoscopic cholecystectomy was 90%, with a range between 71.4% and 100%.
Subgroup analysis
Only one of the trials was at low risk of bias (Sandhu 2009). So this subgroup analysis was not performed. The remaining subgroup analyses were not performed because of the few trials included in the subgroups for the primary outcomes.
Reporting bias
Reporting bias could be assessed for conversion to open cholecystectomy and operating time. Visual inspection of the funnel plot and Egger's linear regression method of assessment of the funnel plot revealed no evidence of reporting bias (conversion to open cholecystectomy: P value 0.50; operating time: P value 0.07).
Discussion
Summary of main results
This review compared the safety and effectiveness of low pressure pneumoperitoneum versus standard pressure pneumoperitoneum. No mortality was noted in either group in the eight trials that reported mortality (Wallace 1997; Dexter 1999; Perrakis 2003; Hasukic 2005; Chok 2006; Karagulle 2008; Sandhu 2009; Celik 2010). Serious adverse events were reported in seven trials only (Wallace 1997; Dexter 1999; Hasukic 2005; Chok 2006; Karagulle 2008; Sandhu 2009; Celik 2010). No statistically significant difference was seen in the proportion of participants with serious adverse events between the low pressure group (1/179; 0.6%) and the standard pressure group (0/215; 0%). Conversion to open cholecystectomy was reported in 10 trials (Dexter 1999; Barczynski 2003; Perrakis 2003; Sefr 2003; Chok 2006; Ibraheim 2006; Karagulle 2008; Joshipura 2009; Sandhu 2009; Celik 2010). In many of these trials, the reason for conversion and the outcomes of participants who underwent conversion to open cholecystectomy were not reported (Barczynski 2003; Perrakis 2003; Sefr 2003; Ibraheim 2006; Joshipura 2009). A small proportion of participants who underwent conversion to open cholecystectomy (and for whom the reason for conversion or the outcome was not available) may have been converted to open cholecystectomy because of procedure‐related injuries such as injuries to the viscera or bile duct. This possibility has not been ruled out in this review. In addition, the confidence intervals of serious adverse events are wide, and significant increases or decreases in complications due to low pressure pneumoperitoneum cannot be ruled out. Hence, no conclusion can be made about the safety of low pressure pneumoperitoneum.
The potential benefit of using low pressure pneumoperitoneum is reduced cardiopulmonary complications. However, even in trials that reported morbidity, no cardiopulmonary complications were described. This is likely to be due to inclusion of only low anaesthetic risk participants in the trials, as well as the low overall incidence of cardiopulmonary complications (0.5% in a case series of 400 patients, 70% of whom were low anaesthetic risk patients) (Dexter 1997). Information on whether low pressure could be beneficial in patients with cardiopulmonary disease is not available from the trials included in this review and requires investigation in further trials.
Operating time was two minutes longer in the low pressure group, and this finding is not clinically significant. Hospital stay was not different between the two groups using the random‐effects model. Although the fixed‐effect model showed significantly shorter hospital stay in the low pressure group than in the standard pressure group, this difference is not clinically significant.
Thus no clinical benefit of low pressure pneumoperitoneum is apparent, and information about its safety is lacking.
Overall completeness and applicability of evidence
Most of the trials included low anaesthetic risk participants undergoing elective laparoscopic cholecystectomy. So the findings of this review are applicable only to low anaesthetic risk patients undergoing elective laparoscopic cholecystectomy.
Quality of the evidence
Only one of the included trials was assessed as having low risk of bias, although it is possible to perform trials with low risk of bias for this comparison as compared with many other comparisons in surgery for which it is not possible to perform trials with low risk of bias (Sandhu 2009). The quality of the evidence is low or very low, as shown in Table 1. However, this is the best quality evidence available on this topic.
Potential biases in the review process
We performed a thorough search of the literature. However, some trials may not have been reported by the researchers because of the lack of benefit associated with low pressure pneumoperitoneum. However, this would not have affected the conclusions of this review in that we do not recommend low pressure pneumoperitoneum unless future trials demonstrate clinical benefit.
Agreements and disagreements with other studies or reviews
We agree with the findings of our previous version that the safety of low pressure pneumoperitoneum has not been established (Gurusamy 2009).
Authors' conclusions
Implications for practice.
Laparoscopic cholecystectomy can be completed successfully using low pressure in approximately 90% of people undergoing laparoscopic cholecystectomy. However, currently no evidence is available to support the use of low pressure pneumoperitoneum. The safety of low pressure pneumoperitoneum has yet to be established.
Implications for research.
Further trials with low risk of bias are required for elective laparoscopic cholecystectomy, laparoscopic cholecystectomy in patients with acute cholecystitis, and laparoscopic cholecystectomy in patients with cardiopulmonary disorders.
Future trials need to be designed according to the SPIRIT guidelines (www.spirit‐statement.org/) and conducted and reported in accordance with the CONSORT statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 29 March 2013 | Amended | Author list: Kurinchi Selvan Gurusamy, Jessica Vaughan, Brian R Davidson. |
| 29 March 2013 | New citation required and conclusions have changed | The methods of the review have been revised according to version 5.1.0 of Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The conclusions now read: "There is currently no evidence to support the use of low pressure pneumoperitoneum. The safety of low pressure pneumoperitoneum has to be established. Further well‐designed trials are necessary". The conclusions in the published 2009 version read: "Low pressure pneumoperitoneum appears effective in decreasing pain after laparoscopic cholecystectomy. The safety of low pressure pneumoperitoneum has to be established". |
| 19 February 2013 | New search has been performed | The search was updated, and nine new trials were included (Karagulle 2008; Kanwer 2009; Sandhu 2009; Joshipura 2009; Torres 2009; Kandil 2010; Celik 2010; Topal 2011; Eryilmaz 2012). |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 29 October 2008 | Amended | Converted to new review format. |
Acknowledgements
To the Cochrane Hepato‐Biliary Group for the support provided.
Peer Reviewers: Yogesh Puri, UK; Rutger Schols, The Netherlands. Contact Editor: Steffano Trastulli, Italy.
K Samraj who identified trials and extracted data for the previous version of this review.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health'.
Appendices
Appendix 1. Search strategies
| Database | Period of Search | Search Strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Wiley) | Issue 1, 2013 | #1 MeSH descriptor Cholecystectomy, Laparoscopic explode all trees #2 (laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) AND cholecystectom* #3 (#1 OR #2) #4 MeSH descriptor Pneumoperitoneum, Artificial explode all trees #5 MeSH descriptor Insufflation explode all trees #6 MeSH descriptor Abdominal Wall explode all trees #7 pneumoperitoneum OR insufflation OR "abdominal wall lift" OR gasless #8 (#4 OR #5 OR #6 OR #7) #9 (#3 AND #8) |
| MEDLINE (PubMed) | 1987 to February 2013 | (laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) AND (cholecystectom* OR cholecystectomy, laparoscopic[MeSH]) AND (pneumoperitoneum OR Pneumoperitoneum, Artificial[MeSH] OR insufflation OR insufflation[MeSH] OR "abdominal wall lift" OR Abdominal Wall[MeSH] OR gasless) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) AND humans [mh]) |
| EMBASE (Ovid SP) | 1987 to February 2013 | 1 exp CROSSOVER PROCEDURE/ 2 exp DOUBLE BLIND PROCEDURE/ 3 exp SINGLE BLIND PROCEDURE/ 4 exp RANDOMIZED CONTROLLED TRIAL/ 5 (((RANDOM* or FACTORIAL* or CROSSOVER* or CROSS) and OVER*) or PLACEBO* or (DOUBL* and BLIND*) or (SINGL* and BLIND*) or ASSIGN* or ALLOCAT* or VOLUNTEER*).af. 6 1 or 2 or 3 or 4 or 5 7 (laparoscop* or coelioscop* or celioscop* or peritoneoscop*).af. 8 "cholecystectom*".af. 9 8 and 7 10 exp Cholecystectomy/ 11 exp Laparoscopic Surgery/ 12 11 and 10 13 9 or 12 14 (pneumoperitoneum or insufflation or "abdominal wall lift" or gasless).af. 15 exp Pneumoperitoneum/ 16 exp Abdominal Wall/ 17 16 or 15 or 14 18 6 and 13 and 17 |
| Science Citation Index Expanded (Web of Knowledge) | 1987 to February 2013 | #1 TS=(laparoscop* OR coelioscop* OR celioscop* OR peritoneoscop*) #2 TS=(cholecystectom*) #3 TS=(pneumoperitoneum OR insufflation OR "abdominal wall lift" OR gasless) #4 TS=(random* OR blind* OR placebo* OR meta‐analysis) #5 #4 AND #3 AND #2 AND #1 |
Data and analyses
Comparison 1. Low pressure versus standard pressure pneumoperitoneum.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 7 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 2 Conversion to open cholecystectomy | 10 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.29, 4.72] |
| 3 Hospital stay | 5 | 415 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.63, 0.02] |
| 4 Operating time | 19 | 990 | Mean Difference (IV, Fixed, 95% CI) | 1.51 [0.07, 2.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barczynski 2002.
| Methods | Randomised clinical trial | |
| Participants | Country: Poland.
Number randomly assigned: 20.
Postrandomisation dropouts: zero (0%). Revised sample size: 20. Mean age: 46 years. Females: 11 (55%). Inclusion criteria: Laparoscopic cholecystectomy due to uncomplicated symptomatic gallstones. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7 mm Hg (n = 10). Group 2: standard pressure 12 mm Hg (n = 10). | |
| Outcomes | None of the outcomes of interest for this review were reported. | |
| Notes | Through their replies in February 2008, the authors of the trial confirmed that this trial was different from Barczynski 2003. Additional attempts to contact the trial authors in March 2013 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "... randomised (closed envelope method) to either LP or SP pneumoperitoneum groups..." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Low risk | Quote: "This work was supported by the grant BBN‐501/KL/456/L from Jagiellonian University College of Medicine." |
Barczynski 2003.
| Methods | Randomised clinical trial. | |
| Participants | Country: Poland.
Number randomly assigned: 148.
Postrandomisation dropouts: zero. Revised sample size: 148. Mean age: 48 years. Females: 129 (87.2%). Inclusion criteria: 1. Uncomplicated, symptomatic cholelithiasis. 2. ASA I or II. 3. Age > = 18 years. Exclusion criteria: 1. Pregnancy and lactation. 2. Previous extensive abdominal surgery. 3. Prolonged administration of NSAIDs or other analgesics. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7 mm Hg (n = 74). Group 2: standard pressure 12 mm Hg (n = 74). | |
| Outcomes | The outcomes reported were conversion to open cholecystectomy, quality of life, hospital stay, and operating time. | |
| Notes | Through their replies in February 2008, the authors of the trial confirmed that this trial was different from Barczynski 2003. Additional attempts to contact the trial authors in March 2013 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization was based on each patient receiving a sealed envelope containing a random number selected from the table assigning the given individual to one of two groups).” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization was based on each patient receiving a sealed envelope containing a random number selected from the table assigning the given individual to one of two groups).” |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “Neither the patients nor the nurses knew the relevant group assignment.” Comment: Assessor blinding of primary outcomes such as surgical morbidity was not performed, and so the blinding is inadequate in this trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Basgul 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey.
Number randomly assigned: 22.
Postrandomisation dropouts: not stated.
Revised sample size: 22. Mean age: 49 years. Females: 10 (45.5%). Inclusion criteria: 1. Undergoing laparoscopic cholecystectomy. 2. Adults. 3. ASA grade I or II. Exclusion criteria: 1. Endocrine or immune system disorders. 2. Malignant or chronic inflammatory disease. 3. Marked obesity 4. Acute cholecystitis. 5. Kidney or liver disorders. 6. Patients on immunosuppressive treatment. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure10 mm Hg (n = 11). Group 2: standard pressure 14 to 15 mm Hg (n = 11). | |
| Outcomes | The outcome reported was operating time. | |
| Notes | Attempts to contact the trial authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Celik 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey.
Number randomly assigned: 100.
Postrandomisation dropouts: zero.
Revised sample size: 100. Mean age: 42 years. Females: 81 (81%). Inclusion criteria: Elective laparoscopic cholecystectomy. Exclusion criteria: 1. Cholangitis, acute cholecystitis, or pancreatitis. 2. Cardiovascular and renal diseases. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 mm Hg or 10 mm Hg (n = 40). Group 2: standard pressure 12 mm Hg or 14 mm Hg or 16 mm Hg (n = 60). | |
| Outcomes | The outcome reported was operating time. | |
| Notes | Attempts to contact the trial authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Celik 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomly assigned: 64. Postrandomisation dropouts: four (6.3%). Revised sample size: 60. Average age: 44 years. Females: 60 (100%). Successful completion of low pressure laparoscopic cholecystectomy: 20/23 (87.0%) Intraoperative cholangiogram: not stated. Inclusion criteria: Female patients with cholelithiasis. Exclusion criteria: 1. American Society of Anesthesiologists physical status grade III or IV. 2. Age younger than 18 years or older than 65 years. 3. Inability to understand the research questionnaire. 4. Having endoscopic retrograde cholangiopancreatography intervention in last 30 days. 5. Undergoing acute cholecystitis attack. 6. Pregnancy. 7. Patients with co‐morbidities like hepatic, renal, endocrine, and immunologic diseases, which can cause chronic pain. 8. Patients who had been using opioids or tranquilising medications for longer than one week before the surgery. 9. Patients with history of drug abuse. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 mm Hg (n = 20). Group 2: standard pressure 12 mm or 14 mm Hg (n = 40). | |
| Outcomes | Outcomes reported were mortality, morbidity, and operating time. | |
| Notes | Attempts to contact the trial authors in March 2013 were unsuccessful. Reasons for postrandomisation dropouts: conversion to open cholecystectomy (one in standard pressure); pericholecystic adhesions (two in low pressure); and increase in pressure from low pressure group to standard pressure group (the participant who underwent conversion to open cholecystectomy was included for conversion to open cholecystectomy but was excluded from other outcomes). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After induction of anesthesia, patients were randomized prospectively into three groups by computer generation." |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Chok 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: China.
Number randomly assigned: 40.
Postrandomisation dropouts: zero.
Revised sample size: 40. Mean age: 47 years. Females: 24 (60%). Inclusion criteria: 1. Symptomatic cholelithiasis with or without complications. 2. Elective outpatient cholecystectomy. 3. ASA I or II. 4. Age < 70 years. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7 mm Hg (n = 20). Group 2: standard pressure 12 mm Hg (n = 20). | |
| Outcomes | Outcomes reported were mortality, morbidity, conversion to open cholecystectomy, and operating time. | |
| Notes | Trial authors provided additional information in February 2008 and March 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence is from random number table (author replies)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed preoperatively at the pre‐anesthetic clinic by drawing consecutive sealed and numbered envelopes by an independent third party (author replies)." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "A preset questionnaire was completed on postoperative days 1 and 3 through telephone by the nursing staff, who were blinded to the randomization results (author replies)." Comment: Assessor blinding of primary outcomes was not performed, and so the blinding is inadequate in this trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Low risk | Quote: "This study was funded by the Tung Wah Group of Hospitals (TWGHs) Research Fund‐Research Project." |
Dexter 1999.
| Methods | Randomised clinical trial. | |
| Participants | Country: United Kingdom.
Number randomly assigned: 23.
Postrandomisation dropouts: three (13%).
Revised sample size: 20. Mean age: 52 years. Females: 13 (65%). Inclusion criteria: 1. Elective laparoscopic cholecystectomy. 2. ASA I or II. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7 mm Hg (n = 10). Group 2: standard pressure 15 mm Hg (n = 10). | |
| Outcomes | Outcomes reported were mortality, morbidity, and conversion to open cholecystectomy. | |
| Notes | Postrandomisation dropouts: two in low pressure group because of cross‐over; one in standard pressure group because of conversion to open cholecystectomy. Trial authors provided additional information in February 2008. Additional attempts to contact the trial authors in March 2013 were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was by computer (author replies)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was drawn when the patient entered the anaesthetic room, by contacting a third person with the envelopes (author replies)." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Three postrandomisation dropouts were reported. These were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Eryilmaz 2012.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomly assigned: 43. Postrandomisation dropouts: not stated. Revised sample size: 43. Average age: 51 years. Females: 26 (60.5%). Successful completion of low‐pressure laparoscopic cholecystectomy: not stated. Intraoperative cholangiogram: not stated. Inclusion criteria: ASA physical status I or II patients undergoing elective laparoscopic cholecystectomy. Exclusion criteria: 1. Patients with liver failure. 2. Coagulopathy. 3. Known allergy to medications. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 10 mm Hg (n = 20). Group 2: standard pressure 14 mm Hg (n = 23). | |
| Outcomes | None of the outcomes included in this review were reported in this trial. | |
| Notes | Attempts to contact the trial authors in March 2013 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Hasukic 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: Bosnia.
Number randomly assigned: 50.
Postrandomisation dropouts: not stated.
Revised sample size: 50. Mean age: 43 years. Females: 45 (90%). Inclusion criteria: 1. Uncomplicated symptomatic cholelithiasis. 2. ASA I or II. 3. Age >= 18 years. 4. No history of previous liver disease. 5. Normal values on preoperative liver function tests. Exclusion criteria: 1. Concomitant common bile duct exploration. 2. Acute cholecystitis. 3. Pregnancy and lactation. 4. Previous extensive abdominal surgery. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7 mm Hg (n = 25). Group 2: standard pressure 14 mm Hg (n = 25). | |
| Outcomes | Outcomes reported were mortality, morbidity, and operating time. | |
| Notes | Trial authors provided additional information in February 2008 and April 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: "The randomization was based on sealed envelopes containing random numbers selected from the table (author replies)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Every patient admitted to the hospital for cholecystectomy who met the inclusive criteria for the study received a sealed envelope with the written method: standard pressure or low pressure. Stated envelopes were opened immediately before laparoscopic cholecystectomy" (author replies). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Low risk | Quote: "The clinical study was performed at the clinic without financial support outside of the University Clinical Center." . |
Ibraheim 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: Saudi Arabia.
Number randomly assigned: 20.
Postrandomisation dropouts: not stated.
Revised sample size: 20. Mean age: 49 years. Females: 14 (70%). Inclusion criteria: Elective laparoscopic cholecystectomy. Exclusion criteria: 1. Respiratory or coronary artery disease. 2. Coagulopathy. 3. Body mass index > 30. 4. Previous gastric surgery. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 6 to 8 mm Hg (n = 10). Group 2: standard pressure 12 to 14 mm Hg (n = 10). | |
| Outcomes | Outcomes reported were conversion to open cholecystectomy and operating time. | |
| Notes | Attempts to contact the trial authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "They were randomly allocated using a sealed envelope method to one of two study groups..." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Joshipura 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: India. Number randomly assigned: 26. Postrandomisation dropouts: zero (0%). Revised sample size: 26. Average age: 57 years. Females: 11 (42.3%). Successful completion of low pressure laparoscopic cholecystectomy: 10/14 (71.4%). Intraoperative cholangiogram: not stated. Inclusion criteria: Patients with uncomplicated symptomatic gall stones were included in the study. Exclusion criteria: 1. Patients with complicated gall stone disease like pyocele or gangrene of gall bladder, acute gall stone pancreatitis, and gall stone with common bile duct stone. 2. History of cholangitis. 3. Gall bladder carcinoma. 4. Patients with previous history of upper abdominal surgery. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 mm Hg (trocar insertion at 12 mm Hg) (n = 14). Group 2: standard pressure 12 mm Hg (n = 12). | |
| Outcomes | Outcomes reported were operating time and hospital stay. | |
| Notes | Attempts to contact the trial authors in March 2013 were unsuccessful. Reasons for postrandomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized to either HPLC or LPLC group just before starting surgery by a picking up closed envelope randomly by a nurse not involved in this study." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients were blinded about their group to avoid bias in postoperative period whereas considering the visual analog scale for pain. Operating surgeons were also blinded about set pressure, as they had to scale at the end of surgery about the vision, space for dissection, and surgical space while doing the suction. However, it was allowed to ‘‘increase the pressure’’ without un‐blinding if surgeon found difficulty in getting adequate space for dissection but it was recorded as a conversion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Kandil 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: Egypt. Number randomly assigned: 116. Postrandomisation dropouts: 16 (13.8%). Revised sample size: 100. Average age: 42 years. Females: 62 (62%). Successful completion of low pressure laparoscopic cholecystectomy: not stated clearly. Intraoperative cholangiogram: not stated. Inclusion criteria: Patients who were treated for gall bladder stone (either elective or having acute cholecystitis). Exclusion criteria: 1. Age > 80 years. 2. History of upper laparotomy. 3. Hemorrhagic tendency due to cirrhosis. 4. American Society of Anesthesiology grade of III or higher. 5. Patients refused to give informed consent. 6. Patients in whom the laparoscopic procedure was converted to open cholecystectomy or from low to high pressure. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 mm or 10 mm Hg (n = 50). Group 2: standard pressure 12 mm Hg or 14 mm Hg (n = 50). | |
| Outcomes | None of the outcomes included in this review were reported in this trial. | |
| Notes | Attempts to contact the trial authors in March 2013 were unsuccessful. Reasons for postrandomisation dropouts: The laparoscopic procedure was converted to open cholecystectomy (two), refused to give informed consent (one), history of upper laparotomy (three), Grade III by American Society of Anesthesiology (two), the laparoscopic procedure was converted from low to higher pneumoperitoneum pressure (four), and participants had cirrhotic liver (four). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Kanwer 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: India. Number randomly assigned: 60. Postrandomisation dropouts: five (8.3%). Revised sample size: 55. Average age: not stated. Females: not stated. Successful completion of low pressure laparoscopic cholecystectomy: 27/30 (90%). Intraoperative cholangiogram: not stated. Inclusion criteria: Patients with uncomplicated symptomatic gall stone disease tagged for laparoscopic cholecystectomy. Exclusion criteria: Patients with acute cholecystitis and with complications of gall stone disease like gall bladder perforation, empyema, and common bile duct stone. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 10 mm Hg (n = 27). Group 2: standard pressure 14 mm Hg (n = 28). | |
| Outcomes | Outcomes reported were mortality and operating time. | |
| Notes | Trial authors provided additional information in March 2013. Reasons for postrandomisation dropouts: conversion to open cholecystectomy (two in standard pressure group) and conversion to standard pressure (from low pressure group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two groups using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelope method (author replies)." Comment: Further details were not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "No blinding (author replies)." Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as morbidity were not reported. |
| For‐profit bias? | Low risk | Quote: "Institute funded (author replies)." |
Karagulle 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomly assigned: 45. Postrandomisation dropouts: one (2.2%). Revised sample size: 44. Average age: 47 years. Females: 37 (84.1%). Successful completion of low pressure laparoscopic cholecystectomy: not stated. Intraoperative cholangiogram: not stated. Inclusion criteria: Patients with ASA I or II with symptomatic cholelithiasis. Exclusion criteria: 1. Patients who had acute cholecystitis. 2. Previous upper abdominal surgery. 3. Systemic or connective tissue diseases. 4. Using tobacco and/or had lung diseases (obstructive, restrictive, or both). | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 mm Hg (n = 14). Group 2: standard pressure 12 mm Hg or 15 mm Hg (n = 30). | |
| Outcomes | Outcomes reported were mortality, morbidity, and conversion to open cholecystectomy. | |
| Notes | Attempts to contact the trial authors in March 2013 were unsuccessful. Reasons for postrandomisation dropouts: conversion to open cholecystectomy (one in low pressure group). This participant was included for conversion to open cholecystectomy but was excluded from other outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Koc 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey.
Number randomly assigned: 53.
Postrandomisation dropouts: three (5.7%).
Revised sample size: 50. Mean age: 47 years. Females: 41 (82%). Inclusion criteria: 1. Elective laparoscopic cholecystectomy for symptomatic gall stones. 2. ASA I to III. Exclusion criteria: 1. Acute cholecystitis or acute pancreatitis. 2. Common bile duct exploration or another surgical procedure. 3. Treatment with drugs that influence neurohormonal parameters. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 10 mm Hg (n = 25). Group 2: standard pressure 15 mm Hg (n = 25). | |
| Outcomes | The outcome reported was operating time. | |
| Notes | Reason for postrandomisation dropout: conversion to open cholecystectomy
(group not stated). Attempts to contact the trial authors in February 2008 were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "...patients were randomized to low‐ or high‐pressure pneumoperitoneum groups by closed envelope method in the operating room prior to surgery." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "...a short‐form MGQ (SF‐MGQ) was obtained from all patients by a research assistant who was blind to the group allocation of the patients." Comment: Assessor blinding of primary outcomes was not performed, and so the blinding is inadequate in this trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Three postrandomisation dropouts were due to conversion to open cholecystectomy. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Perrakis 2003.
| Methods | Randomised clinical trial. | |
| Participants | Country: Greece.
Number randomly assigned: 40.
Postrandomisation dropouts: zero.
Revised sample size: 40. Mean age: 57 years. Females: 30 (75%). Inclusion criteria: 1. Elective laparoscopic cholecystectomy. 2. ASA I or II. Exclusion criteria: 1. Regular analgesic medication. 2. Extensive upper abdominal incision. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 mm Hg (n = 20). Group 2: standard pressure 15 mm Hg (n = 20). | |
| Outcomes | Outcomes reported were mortality, conversion to open cholecystectomy, and operating time. | |
| Notes | Attempts to contact the trial authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed preoperatively using envelopes with random numbers." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Pier 1994.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany.
Number randomly assigned (included for this review): 22.
Postrandomisation dropouts: not stated.
Revised sample size: 22. Mean age: not stated. Females: not stated. Inclusion criteria: 1. Elective laparoscopic cholecystectomy. 2. Age > 18 years. 3. ASA I or II. Exclusion criteria: Conversion to open cholecystectomy. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure < 9 mm Hg (n = 12). Group 2: standard pressure 14 to 16 mm Hg (n = 10). Two other groups (high pressure > 18 mm Hg and standard pressure 14 to 16 mm Hg with Argon gas) were excluded from this review. |
|
| Outcomes | The outcome reported was operating time. | |
| Notes | Attempts to contact the trial authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Polat 2003.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey.
Number randomly assigned: 24.
Postrandomisation dropouts: not stated.
Revised sample size: 24. Mean age: 48 years. Females: 11 (45.8%). Inclusion criteria: Laparoscopic cholecystectomy for symptomatic cholelithiasis. Exclusion criteria: Patients with metabolic, endocrine, hepatic, or renal disease. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 10 mm Hg (n = 12). Group 2: standard pressure 15 mm Hg (n = 12). | |
| Outcomes | The outcome reported was operating time. | |
| Notes | Attempts to contact the study authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Sandhu 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: Thailand. Number randomly assigned: 140. Postrandomisation dropouts: zero (0%). Revised sample size: 140. Average age: 55 years. Females: 111 (79.3%). Successful completion of low pressure laparoscopic cholecystectomy: 68/70 (97.1%). Intraoperative cholangiogram: not stated. Inclusion criteria: 1. Adults with American Society of Anesthesiologists (ASA) physical status grade I or II. 2. Undergoing elective laparoscopic cholecystectomy for benign gall bladder. Exclusion criteria: 1. Patients younger than 18 years. 2. Those who refused to give consent. 3. Inability to understand the research questionnaire. 4. Pregnancy. 5. Patients who were found to require additional procedures at surgery. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7 mm Hg (n = 70). Group 2: standard pressure 14 mm Hg (n = 70). | |
| Outcomes | Outcomes reported were mortality, morbidity, conversion to open cholecystectomy, operating time, and hospital stay. | |
| Notes | Study authors provided additional information in March 2013. Reasons for postrandomisation dropouts: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After induction of anesthesia, patients were randomized prospectively by computer generation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Put a coded card (label pressure 7 mmHg or 14 mmHg as prospectively randomized by computer generation) in an opaque, sealed, and sequentially numbered envelope. Then open the envelope before surgery by surgeon (author replies)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "both the patients and healthcare providers were blinded. The outcome assessors were blinded as well (author replies)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Low risk | Quote: "This study was not supported by any funding sources. We used our own budget (author replies)." |
Sefr 2003.
| Methods | Randomised clinical trial. | |
| Participants | Country: Czech Republic.
Number randomly assigned: 30.
Postrandomisation dropouts: zero.
Revised sample size: 30. Mean age: 54 years. Females: 23 (76.7%). Inclusion criteria: 1. Laparoscopic cholecystectomy for uncomplicated symptomatic gall stone disease. 2. ASA I or II. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 10 mm Hg (n = 15). Group 2: standard pressure 15 mm Hg (n = 15). | |
| Outcomes | Outcomes reported were conversion to open cholecystectomy and operating time. | |
| Notes | The study authors provided additional information in February 2008. Additional attempts to contact the study authors in March 2013 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was achieved by computerized random‐number generation." |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation was done by a third party ‐ university dept. of statistics (author replies)." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Topal 2011.
| Methods | Randomised clinical trial. | |
| Participants | Country: Turkey. Number randomly assigned: 60. Postrandomisation dropouts: not stated. Revised sample size: 60. Average age: 42 years. Females: 13 (21.7%). Successful completion of low pressure laparoscopic cholecystectomy: not stated. Intraoperative cholangiogram: not stated. Inclusion criteria: 1. Patients who underwent laparoscopic surgery for non‐complicated cholelithiasis. Exclusion criteria: 1. Patients with preexisting coagulation derangement. 2. Acute cholecystitis, cholangitis, or other acute inflammation. 3. Current or recent (six months) acute pancreatitis. 4. Haematological disorders. 5. Anticoagulant treatment. 6. Current or recent (six months) thromboembolic disorders. 7. Renal, hepatic, rheumatic, or vascular disease. 8. Recent (six months) surgery. 9. Current or recent (three years) malignancy. 10. Pregnancy. 11. Positive family history for coagulation disorders. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 10 mm Hg (n = 20). Group 2: standard pressure 13 mm or 16 mm Hg (n = 40). | |
| Outcomes | The outcome reported was operating time. | |
| Notes | Attempts to contact the study authors in March 2013 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Torres 2009.
| Methods | Randomised clinical trial. | |
| Participants | Country: Poland. Number randomly assigned: 40. Postrandomisation dropouts: not stated. Revised sample size: 40. Average age: 49 years. Females: 28 (70%). Successful completion of low pressure laparoscopic cholecystectomy: not stated. Intraoperative cholangiogram: not stated. Inclusion criteria: Patients with cholelithiasis and indications for surgical treatment. Exclusion criteria: 1. No symptoms of acute cholecystitis. 2. Diabetes. 3. Body mass index. 4. Above 36 kg/m2. 5. Autoimmunological diseases. | |
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 6 to 8 mm Hg (n = 20). Group 2: standard pressure 12 to 14 mm Hg (n = 20). | |
| Outcomes | The outcome reported was operating time. | |
| Notes | Attempts to contact the trial authors in March 2013 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Unbehaum 1995.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany.
Number randomly assigned: 71.
Postrandomisation dropouts: 10 (14.1%).
Revised sample size: 61. Mean age: 47 years. Females: 44 (72.1%). Inclusion criteria: 1. Elective laparoscopic cholecystectomy. 2. Symptomatic gall stones. Exclusion criteria: 1. Conversion to open cholecystectomy. 2. Requirement for other procedures. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 8 to 10 mm Hg (n = 30). Group 2: standard pressure 14 to 16 mm Hg (n = 31). | |
| Outcomes | The outcome reported was hospital stay. | |
| Notes | Reason for postrandomisation dropouts was not stated. We were unable to contact the trial authors in December 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Die Vorlage der Analog skalzt erfolgte im Blindversuch, das heißt durch eine Person, der nicht bekannt war, welcher der beiden Versuchsgrttppen der Patient zugeordnet worden war" (Translation: pain scores were recorded by a person who was not aware of patient groups). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as mortality and morbidity were not reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Wallace 1997.
| Methods | Randomised clinical trial. | |
| Participants | Country: United Kingdom.
Number randomly assigned: 40.
Postrandomisation dropouts: zero.
Revised sample size: 40. Mean age: 58 years. Females: 30 (75%). Inclusion criteria: Laparoscopic cholecystectomy for uncomplicated symptomatic gall stone disease. |
|
| Interventions | Participants were randomly assigned to different pressures of pneumoperitoneum. Group 1: low pressure 7.5 mm Hg (n = 20). Group 2: standard pressure 15 mm Hg (n = 20). | |
| Outcomes | Outcomes reported were mortality, morbidity, hospital stay, and operating time. | |
| Notes | Attempts to contact the study authors in February 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: "Randomization was by computerized random‐number generation." |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: "the patient, investigator and senior registrar who discharged the patients were blind to the operating insufflation pressure." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No postrandomisation dropouts were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes such as mortality and morbidity were reported. |
| For‐profit bias? | Unclear risk | Comment: This information was not available. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barczynski 2004 | Study of effect of saline washout during low pressure laparoscopic cholecystectomy. |
| Beqiri 2012 | Neither the number of ports nor the size of the ports was reported in this trial. |
| Brokelman 2006 | The size of the ports was not stated. |
| Esmat 2006 | Quasi‐randomised trial (systematic randomisation). |
| Giraudo 2001 | Neither the number of ports nor the size of the ports was reported in this trial. |
| Morino 1998 | Neither the number of ports nor the size of the ports was reported in this trial. |
| Parikh 2009 | Not a randomised clinical trial. |
| Sandoval‐Jimenez 2009 | The size of ports in the control group was not stated. |
| Sarli 2000 | Quasi‐randomised trial (randomisation by drawing cards labelled A and B). |
| Tou 2004 | Comment on an included trial (Sefr 2003). |
| Yasir 2012 | Neither the number of ports nor the size of the ports was reported in this trial. |
Differences between protocol and review
Differences between first and second versions
The methods of the review have been revised according to version 5.1.0 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This resulted in a change in outcomes and in risk of bias. Outcomes related to pain scores were excluded from the revised version, as the clinical implication of reduction in pain is not clear. A clinically significant reduction in pain score is likely to result in shorter hospital stay, earlier return to normal activity, and earlier return to work, all of which have been included in the revised version.
Contributions of authors
KS Gurusamy wrote the review, assessed the trials for inclusion, and extracted data on included trials. J Vaughan identified trials and extracted data on included trials for this version. BR Davidson provided advice on improving the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Barczynski 2002 {published data only}
- Barczynski M, Herman RM. Influence of different pressures of pneumoperitoneum on the autonomic system function during laparoscopy. Folia Medica Cracoviensia 2002;43(1‐2):51‐8. [PubMed] [Google Scholar]
Barczynski 2003 {published data only}
- Barczynski M, Herman RM. A prospective randomized trial on comparison of low‐pressure (LP) and standard‐pressure (SP) pneumoperitoneum for laparoscopic cholecystectomy. Surgical Endoscopy 2003;17(4):533‐8. [DOI] [PubMed] [Google Scholar]
Basgul 2004 {published data only}
- Basgul E, Bahadir B, Celiker V, Karagoz AH, Hamaloglu E, Aypar U. Effects of low and high intra‐abdominal pressure on immune response in laparoscopic cholecystectomy. Saudi Medical Journal 2004;25(12):1888‐91. [PubMed] [Google Scholar]
Celik 2004 {published data only}
- Celik V, Salihoglu Z, Demiroluk S, Unal E, Yavuz N, Karaca S, et al. Effect of intra‐abdominal pressure level on gastric intramucosal pH during pneumoperitoneum. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2004;14(5):247‐9. [DOI] [PubMed] [Google Scholar]
Celik 2010 {published data only}
- Celik AS, Firat N, Celebi F, Guzey D, Kaplan R, Birol S, et al. Laparoscopic cholecystectomy and postoperative pain: is it affected by intra‐abdominal pressure?. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2010;20(4):220‐2. [DOI] [PubMed] [Google Scholar]
Chok 2006 {published data only}
- Chok KS, Yuen WK, Lau H, Fan ST. Prospective randomized trial on low‐pressure versus standard‐pressure pneumoperitoneum in outpatient laparoscopic cholecystectomy. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2006;16(6):383‐6. [DOI] [PubMed] [Google Scholar]
Dexter 1999 {published data only}
- Dexter SP, Vucevic M, Gibson J, McMahon MJ. Hemodynamic consequences of high‐ and low‐pressure capnoperitoneum during laparoscopic cholecystectomy. Surgical Endoscopy 1999;13(4):376‐81. [DOI] [PubMed] [Google Scholar]
Eryilmaz 2012 {published data only}
- Eryilmaz HB, Memis D, Sezer A, Inal MT. The effects of different insufflation pressures on liver functions assessed with LiMON on patients undergoing laparoscopic cholecystectomy. The Scientific World Journal 2012;2012:172575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasukic 2005 {published data only}
- Hasukic S. Postoperative changes in liver function tests ‐ Randomized comparison of low‐ and high‐pressure laparoscopic cholecystectomy. Surgical Endoscopy 2005;19(11):1451‐5. [DOI] [PubMed] [Google Scholar]
Ibraheim 2006 {published data only}
- Ibraheim OA, Samarkandi AH, Alshehry H, Faden A, Farouk EO. Lactate and acid base changes during laparoscopic cholecystectomy. Middle East Journal of Anesthesiology 2006;18(4):757‐68. [PubMed] [Google Scholar]
- Ibraheim OA, Samarkandy AH, Alshehry H, Faden A, Elfarouk EO. Lactate levels and acid‐base equilibrium in high‐ and low‐pressure CO2 pneumoperitoneum for laparoscopic cholecystectomy. Egyptian Journal of Anaesthesia 2005;21(4):337‐40. [Google Scholar]
Joshipura 2009 {published data only}
- Joshipura VP, Haribhakti SP, Patel NR, Naik RP, Soni HN, Patel B, et al. A prospective randomized, controlled study comparing low pressure versus high pressure pneumoperitoneum during laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2009;19(3):234‐40. [DOI] [PubMed] [Google Scholar]
Kandil 2010 {published data only}
- Kandil TS, Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. Journal of Laparoendoscopic and Advanced Surgical Techniques. Part A 2010;20(8):677‐82. [DOI] [PubMed] [Google Scholar]
Kanwer 2009 {published data only}
- Kanwer DB, Kaman L, Nedounsejiane M, Medhi B, Verma GR, Bala I. Comparative study of low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy—a randomised controlled trial. Tropical Gastroenterology 2009;30(3):171‐4. [PubMed] [Google Scholar]
Karagulle 2008 {published data only}
- Karagulle E, Turk E, Dogan R, Ekici Z, Moray G. The effects of different abdominal pressures on pulmonary function test results in laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2008;18(4):329‐33. [DOI] [PubMed] [Google Scholar]
Koc 2005 {published data only}
- Koc M, Ertan T, Tez M, Kocpinar MA, Kilic M, Gocmen E, et al. Randomized, prospective comparison of postoperative pain in low‐versus high‐pressure pneumoperitoneum. Australian and New Zealand Journal of Surgery 2005;75(8):693‐6. [DOI] [PubMed] [Google Scholar]
Perrakis 2003 {published data only}
- Perrakis E, Vezakis A, Velimezis G, Savanis G, Deverakis S, Antoniades J, et al. Randomized comparison between different insufflation pressures for laparoscopic cholecystectomy. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2003;13(4):245‐9. [DOI] [PubMed] [Google Scholar]
Pier 1994 {published data only}
- Pier A, Benedic M, Mann B, Buck V. Post‐laparoscopic pain syndrome ‐ results of a prospective randomized study. Der Chirurg 1994;65(3):200‐8. [PubMed] [Google Scholar]
Polat 2003 {published data only}
- Polat C, Yilmaz S, Serteser M, Koken T, Kahraman A, Dilek ON. The effect of different intraabdominal pressures on lipid peroxidation and protein oxidation status during laparoscopic cholecystectomy. Surgical Endoscopy 2003;17(11):1719‐22. [DOI] [PubMed] [Google Scholar]
Sandhu 2009 {published data only}
- Sandhu T, Yamada S, Ariyakachon V, Chakrabandhu T, Chongruksut W, Ko‐Iam W. Low‐pressure pneumoperitoneum versus standard pneumoperitoneum in laparoscopic cholecystectomy, a prospective randomized clinical trial. Surgical Endoscopy 2009;23(5):1044‐7. [DOI] [PubMed] [Google Scholar]
Sefr 2003 {published data only}
- Sefr R, Puszkailer K, Frána J, Penka I. [Effect of carbon dioxide pneumoperitoneum on selected parameters of the acid‐base equilibrium in laparoscopic cholecystectomy]. Rozhledy v Chirurgii 2001;80(4):206‐12. [PubMed] [Google Scholar]
- Sefr R, Puszkailer K, Jagos F. Randomized trial of different intraabdominal pressures and acid‐base balance alterations during laparoscopic cholecystectomy. Surgical Endoscopy 2003;17(6):947‐50. [DOI] [PubMed] [Google Scholar]
Topal 2011 {published data only}
- Topal A, Celik JB, Tekin A, Yuceaktas A, Otelcioglu S. The effects of 3 different intra‐abdominal pressures on the thromboelastographic profile during laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2011;21(6):434‐8. [DOI] [PubMed] [Google Scholar]
Torres 2009 {published data only}
- Torres K, Torres A, Staskiewicz GJ, Chroscicki A, Lo T, Maciejewski R. A comparative study of angiogenic and cytokine responses after laparoscopic cholecystectomy performed with standard‐ and low‐pressure pneumoperitoneum. Surgical Endoscopy 2009;23(9):2117‐23. [DOI] [PubMed] [Google Scholar]
Unbehaum 1995 {published data only}
- Unbehaum N, Feussner H, Siewert J R. Low‐pressure insufflation technique in the laparoscopic cholecystectomy. Minimal Invasive Chirurgie 1995;4:10‐5. [Google Scholar]
Wallace 1997 {published data only}
- Wallace DH, Serpell MG, Baxter JN, O'Dwyer PJ. Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. British Journal of Surgery 1997;84(4):455‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Barczynski 2004 {published data only}
- Barczynski M, Herman RM. Low‐pressure pneumoperitoneum combined with intraperitoneal saline washout for reduction of pain after laparoscopic cholecystectomy: a prospective randomized study. Surgical Endoscopy 2004;18(9):1368‐73. [DOI] [PubMed] [Google Scholar]
Beqiri 2012 {published data only}
- Beqiri AI, Domi RQ, Sula HH, Zaimi EQ, Petrela EY. The combination of infiltrative bupivacaine with low‐pressure laparoscopy reduces postcholecystectomy pain. A prospective randomized controlled study. Saudi Medical Journal 2012;33(2):134‐8. [PubMed] [Google Scholar]
Brokelman 2006 {published data only}
- Brokelman WJA, Holmdahl L, Bergstrom M, Falk P, Klinkenbijl JHG, Reijnen M. Peritoneal fibrinolytic response to various aspects of laparoscopic surgery: a randomized trial. Journal of Surgical Research 2006;136(2):309‐13. [DOI] [PubMed] [Google Scholar]
- Brokelman WJA, Holmdahl L, Bergstrom M, Falk P, Klinkonbijl JHG, Reijnen MMPJ. Peritoneal transforming growth factor beta‐1 expression during laparoscopic surgery: a clinical trial. Surgical Endoscopy 2007;21(9):1537‐41. [DOI] [PubMed] [Google Scholar]
Esmat 2006 {published data only}
- Esmat ME, Elsebae MMA, Nasr MMA, Elsebaie SB. Combined low pressure pneumoperitoneum and intraperitoneal infusion of normal saline for reducing shoulder tip pain following laparoscopic cholecystectomy. World Journal of Surgery 2006;30(11):1969‐73. [DOI] [PubMed] [Google Scholar]
Giraudo 2001 {published data only}
- Giraudo G, Brachet Contul R, Caccetta M, Morino M. Gasless laparoscopy could avoid alterations in hepatic function. Surgical Endoscopy 2001;15(7):741‐6. [DOI] [PubMed] [Google Scholar]
Morino 1998 {published data only}
- Morino M, Giraudo G, Festa V. Alterations in hepatic function during laparoscopic surgery. An experimental clinical study. Surgical Endoscopy 1998;12(7):968‐72. [DOI] [PubMed] [Google Scholar]
Parikh 2009 {published data only}
- Parikh H, Mehta M. A study of QT interval and QT dispersion during laparoscopic cholecystectomy. Indian Journal of Anaesthesia 2009;53(2):193‐6. [PMC free article] [PubMed] [Google Scholar]
Sandoval‐Jimenez 2009 {published data only}
- Sandoval‐Jimenez C, Mendez‐Sashida G, Cruz‐Marquez‐Rico L, Cardenas‐Victorica R, Guzman‐Esquivel H, Luna‐Silva M, et al. [Postoperative pain in patients undergoing elective laparoscopic cholecystectomy with low versus standard‐pressure pneumoperitoneum. A randomized clinical trial.]. Revista de Gastroenterologia de Mexico 2009;74(4):314‐20. [PubMed] [Google Scholar]
Sarli 2000 {published data only}
- Sarli L, Costi R. Prospective randomized trial of low‐pressure pneumoperitoneum for reduction of shoulder‐tip pain following laparoscopy. British Journal of Surgery 2000;87(9):1161‐5. [DOI] [PubMed] [Google Scholar]
Tou 2004 {published data only}
- Tou S, Singh‐Ranger D. Randomized trial of different Intraabdominal pressures and acid‐base balance alterations during laparoscopic cholecystectomy. Surgical Endoscopy 2004;18(5):872. [DOI] [PubMed] [Google Scholar]
Yasir 2012 {published data only}
- Yasir M, Mehta KS, Banday VH, Aiman A, Masood I, Iqbal B. Evaluation of post operative shoulder tip pain in low pressure versus standard pressure pneumoperitoneum during laparoscopic cholecystectomy. The Surgeon 2012;10(2):71‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Alijani 2004
- Alijani A, Hanna GB, Cuschieri A. Abdominal wall lift versus positive‐pressure capnoperitoneum for laparoscopic cholecystectomy ‐ randomized controlled trial. Annals of Surgery 2004;239(3):388‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
ASA 2007
- American Association of Anesthiologists. ASA Physical Status Classification System. www.asahq.org/clinical/physicalstatus.htm (accessed 7 August 2013).
Attili 1995
- Attili AF, Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995;21(3):655‐60. [DOI] [PubMed] [Google Scholar]
Ballal 2009
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surgical Endoscopy 2009;23(10):2338‐44. [DOI] [PubMed] [Google Scholar]
Bates 1992
- Bates T, Harrison M, Lowe D, Lawson C, Padley N. Longitudinal study of gall stone prevalence at necropsy. Gut 1992;33(1):103‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2000
- Berger MY, Velden JJ, Lijmer JG, Kort H, Prins A, Bohnen AM. Abdominal symptoms: do they predict gallstones? A systematic review. Scandinavian Journal of Gastroenterology 2000;35(1):70‐6. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive—Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Casati 1997
- Casati A, Valentini G, Ferrari S, Senatore R, Zangrillo A, Torri G. Cardiorespiratory changes during gynaecological laparoscopy by abdominal wall elevation: comparison with carbon dioxide pneumoperitoneum. British Journal of Anaesthesia 1997;78(1):51‐4. [DOI] [PubMed] [Google Scholar]
Cordoba 2010
- Cordoba G, Schwartz L, Woloshin S, Bae H, Gotzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341:c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ 2011 (accessed 7 August 2013).
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dexter 1997
Dolan 2009
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997‐2006. Journal of Gastrointestinal Surgery 2009;13(12):2292‐301. [DOI] [PubMed] [Google Scholar]
Egawa 2006
- Egawa H, Morita M, Yamaguchi S, Nagao M, Iwasaki T, Hamaguchi S, et al. Comparison between intraperitoneal CO2 insufflation and abdominal wall lift on QT dispersion and rate‐corrected QT dispersion during laparoscopic cholecystectomy. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 2006;16(2):78‐81. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galizia 2001
- Galizia G, Prizio G, Lieto E, Castellano P, Pelosio L, Imperatore V, et al. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum and abdominal wall‐lifting cholecystectomy. A prospective, randomized study. Surgical Endoscopy 2001;15(5):477‐83. [DOI] [PubMed] [Google Scholar]
Giger 2006
- Giger UF, Michel JM, Opitz I, Inderbitzin DT, Kocher T, Krahenbuhl L. Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. Journal of American College of Surgeons 2006;203(5):723‐8. [DOI] [PubMed] [Google Scholar]
Giger 2011
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. The British Journal of Surgery 2011;98(3):391‐6. [DOI] [PubMed] [Google Scholar]
Glasgow 2000
- Glasgow RE, Cho M, Hutter MM, Mulvihill SJ. The spectrum and cost of complicated gallstone disease in California. Archives of Surgery 2000;135(9):1021‐5; discussion 1025‐7. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 7. Art. No.: LIVER.
GREPCO 1984
- GREPCO. Prevalence of gallstone disease in an Italian adult female population. Rome group for the epidemiology and prevention of cholelithiasis (GREPCO). American Journal of Epidemiology 1984;119(5):796‐805. [PubMed] [Google Scholar]
GREPCO 1988
- GREPCO. The epidemiology of gallstone disease in Rome, Italy. Part i. Prevalence data in men. The Rome group for epidemiology and prevention of cholelithiasis (GREPCO). Hepatology 1988;8(4):904‐6. [PubMed] [Google Scholar]
Gurusamy 2012
- Gurusamy KS, Koti R, Samraj K, Davidson BR. Abdominal lift for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD006574.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Vaughan J, Ramamoorthy R, Fusai G, Davidson BR. Miniports versus standard ports for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006804.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2014a
- Gurusamy KS, Vaughan J, Toon C, Davidson BR. Pharmacological interventions for prevention or treatment of post‐operative pain in patients undergoing laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue (under editorial). [DOI: 10.1002/14651858.CD008261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2014b
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrin GP, Davidson BR. Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007049.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2014c
- Gurusamy KS, Nagendran M, Guerrini GP, Toon C, Zinnuroglu M, Davidson BR. Intraperitoneal local anaesthetic instillation versus no intraperitoneal local anaesthetic instillation for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007337.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2014d
- Gurusamy KS, Vaughan J, Rossi M, Davidson BR. Fewer‐than‐four ports versus four ports for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD007109.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halldestam 2004
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. [DOI] [PubMed] [Google Scholar]
Henny 2005
- Henny CP, Hofland J. Laparoscopic surgery: pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surgical Endoscopy 2005;19(9):1163‐71. [DOI] [PubMed] [Google Scholar]
HES 2011
- HESonline. Hospital Episode Statistics. Main procedures and interventions: 3 character. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=205 2011 (accessed on 7 August 2013).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Junghans 1997
- Junghans T, Bohm B, Grundel K, Schwenk W. Effects of pneumoperitoneum with carbon dioxide, argon, or helium on hemodynamic and respiratory function. Archives of Surgery 1997;132(3):272‐8. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mertens 2004
- Mertens zur Borg IR, Lim A, Verbrugge SJ, Jzermans IJ, Klein J. Effect of intraabdominal pressure elevation and positioning on hemodynamic responses during carbon dioxide pneumoperitoneum for laparoscopic donor nephrectomy: a prospective controlled clinical study. Surgical Endoscopy 2004;18(6):919‐23. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Neudecker 2002
- Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surgical Endoscopy 2002;16(7):1121‐43. [DOI] [PubMed] [Google Scholar]
Neuhaus 2001
- Neuhaus SJ, Watson DI, Ellis T, Lafullarde T, Jamieson GG, Russell WJ. Metabolic and immunologic consequences of laparoscopy with helium or carbon dioxide insufflation: a randomized clinical study. Australian and New Zealand Journal of Surgery 2001;71(8):447‐52. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NIH 1992
- NIH. NIH consensus statement on gallstones and laparoscopic cholecystectomy. http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm 1992 (accessed 7 August 2013). [PubMed]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Russell 1993
- Russell RC. General surgery: biliary surgery. BMJ (Clinical Research Ed.) 1993;307(6914):1266‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Strasberg 1993
- Strasberg SM, Clavien PA. Overview of therapeutic modalities for the treatment of gallstone diseases. American Journal of Surgery 1993;165(4):420‐6. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 7 August 2013).
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2009
- Gurusamy KS, Samraj K, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006930.pub2] [DOI] [PubMed] [Google Scholar]


