FIGURE 1.
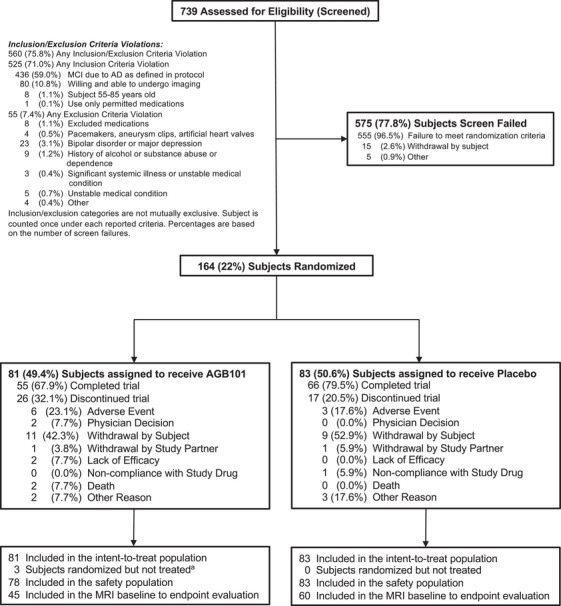
Screening, randomization, and follow‐up of participants in the Hope4MCI study. Participants who completed the week 78 visit were considered to have completed the trial. The intention‐to‐treat population included all participants randomized to treatment who completed a primary endpoint assessment at baseline and at least one dose of AGB101. Three participants were randomized to AGB101 but withdrew from the study before initiating treatment. Reasons for discontinuation included physician decision (n = 1), withdrawal by subject (n = 1), and other (n = 1)
