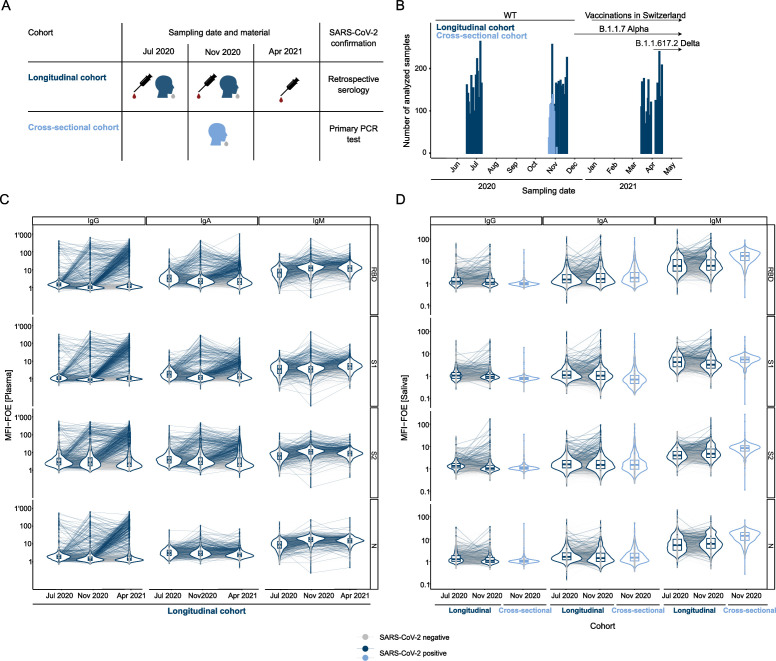
Fig 1.
Multifactorial seroprofiling in a longitudinal cohort of children and a cross-sectional diagnostic cohort. (A) Schematic of serology sampling during three sampling rounds in the longitudinal cohort and one round in the cross-sectional cohort. Information is provided on the type of sampling for serology studies (plasma or saliva). (B) Sampling dates of the longitudinal (dark blue) and cross-sectional (light blue) cohorts. (C) Antibody measurements in the multiplex SARS-CoV-2 ABCORA in plasma in children from the longitudinal cohort with serology measurements at all three visit rounds (n = 1,967). Depicted are median fluorescence intensity (MFI) signals normalized for empty bead controls (fold over empty beads, MFI-FOE). Individuals in light gray stayed seronegative throughout the three visit rounds. Individuals in blue showed seroconversion. (D) Antibody measurements in the multiplex SARS-CoV-2 ABCORA in saliva on all children from the longitudinal cohort with serology measurements at the first two visit rounds (n = 2,806) and on individuals from the cross-sectional cohort (n = 882). SARS-CoV-2 positivity is determined by blood seropositivity in the longitudinal cohort (dark blue) or PCR positivity in the cross-sectional cohort (light blue). SARS-CoV-2 negative individuals (serology or PCR) are depicted in light gray.