Figure 4.
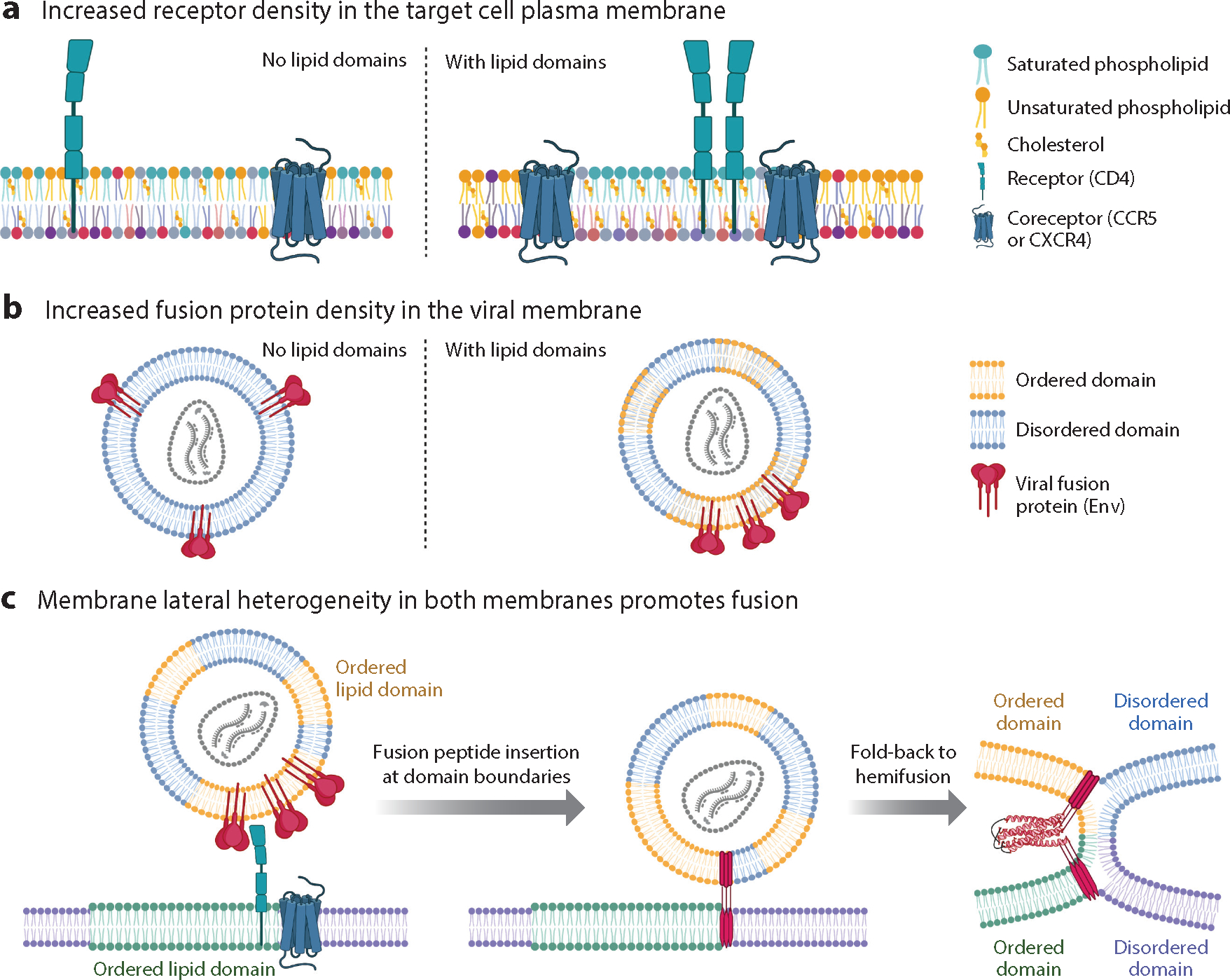
Effects of membrane lateral heterogeneity on viral membrane fusion as exemplified by human immunodeficiency virus (HIV). (a) In target cell membranes, lipid nanodomains can organize and concentrate receptors and/or fusion triggers. For HIV, the receptor, CD4, partitions to ordered nanodomains [rich in saturated (teal) phospholipids and cholesterol] while the HIV coreceptor, CCR5 (fusion trigger), partitions to the domain boundary. (b) In the viral membrane, lipid nanodomains influence the spacing of fusion proteins. On immature HIV particles, envelope (Env) is relatively immobile. Upon maturation via proteolytic cleavage of the juxtamembrane Gag polyprotein, Env diffuses more rapidly to form clusters that facilitate fusion. Env has multiple sequences that promote association with ordered nanodomains (yellow phospholipids; cholesterol not depicted) including a cholesterol recognition amino acid consensus motif within the membrane proximal external region (MPER) and palmitoylation sites, and an additional cholesterol interacting domain in the cytoplasmic tail of gp41. Env partitioning to ordered domains might be cell type and HIV strain dependent. (c) Lateral heterogeneity affects the energetics of fusion. The fusion peptides in the gp41 prehairpin preferentially insert at discontinuities in bilayer thickness between ordered and disordered lipid nanodomains. Upon fusion, the joining of two ordered domains produces one larger domain with a lower ratio of perimeter/area than the starting smaller domains. This minimizes line tension at the domain boundary and contributes favorably to the energetics of fusion. Figure adapted from images created with BioRender.com. See Supplemental Figure Legend 4 for more information and references.
