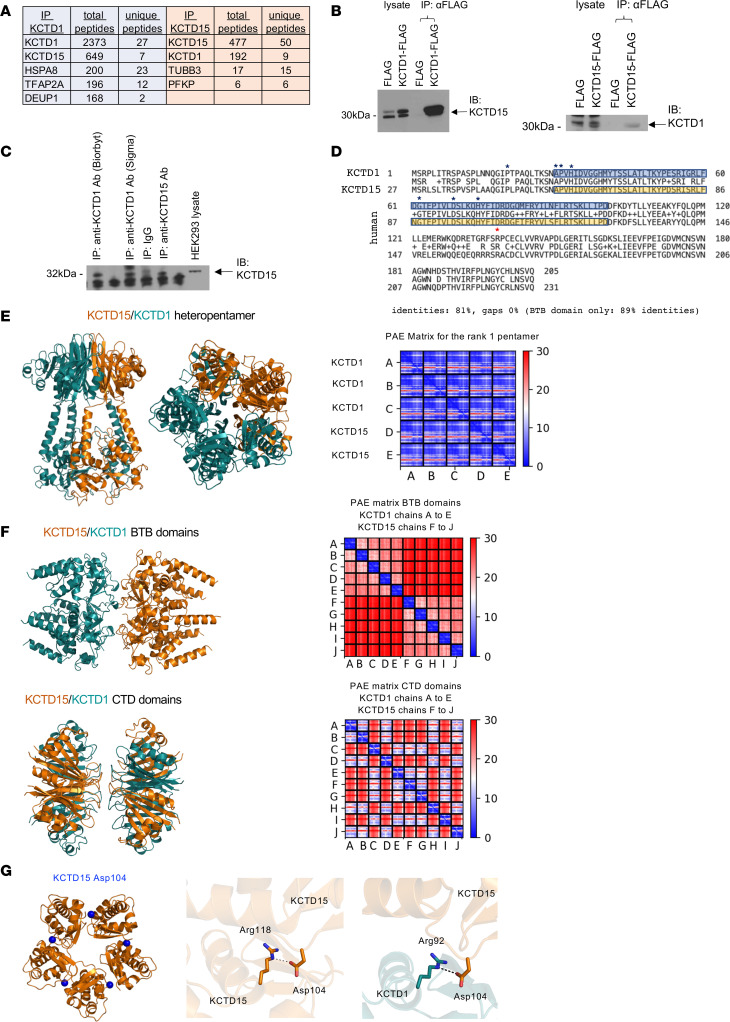
Figure 1. KCTD1 can form heteropentamers with KCTD15.
(A) Proteins with the highest number of total peptides identified by mass spectrometry of immunoprecipitates (IP) with anti-FLAG antibodies using HEK293 cells that overexpress KCTD1-FLAG (IP KCTD1) or KCTD15-FLAG (IP KCTD15). (B) Left: IP of KCTD1-FLAG and immunoblotting for KCTD15. Right: IP of KCTD15-FLAG and immunoblotting for KCTD1. (C) Immunoblotting for KCTD15 of HEK293 cell lysates using KCTD1 or KCTD15 antibodies for IP. (D) Human KCTD1 and KCTD15 amino acid sequences show a high sequence identity, especially in the BTB domain (blue/yellow). Blue stars, amino acids affected by SEN syndrome mutations in KCTD1; red star, amino acid affected by KCTD15 mutation. (E) Left: AlphaFold model of the KCTD1 (green)/KCTD15 (orange) heteropentamer. Right: Reliability of AlphaFold predictions assessed by the predicted aligned error (PAE) matrix, which provides a distance error for every pair of residues within the complex. Blue color, associated with low estimated errors of the distance of pairs of residues, is a strong indication of the global reliability of the model. These data support that KCTD1 and KCTD15 can form stable pentamers in any stoichiometry (here pentamer formation predicted to be stable between 3 KCTD1 and 2 KCTD15 monomers). (F) Top: Reliability of AlphaFold predictions for binding between 5 KCTD1 BTB domains (homomers) and 5 KCTD15 BTB domains (homomers) assessed by the PAE matrix, demonstrating that such an interaction would be unstable. Bottom: AlphaFold predictions for the association of 5 KCTD1 C-terminal domains and 5 KCTD15 C-terminal domains show that the tendency to form heteromers is greater than the tendency to form homomers (e.g., chain A forms non-red boxes with itself, and with chains B, D, H, and J, predicting heteromer formation rather than homomer formation). (G) Left: Asp104 (blue) at the intersubunit interface of the KCTD15 pentamer (BTB domains). Middle: Asp104 forms an intersubunit salt bridge with Arg118 between KCTD15 subunits. Right: Similar electrostatic interaction formed by Asp104 (KCTD15) and Arg92 (KCTD1), the residue that is equivalent to Arg118 of KCTD15, observed at the KCTD1-KCTD15 interface of the heteropentamer.