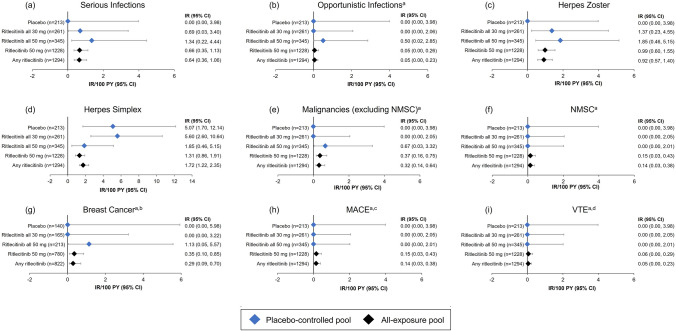
Fig. 2.
IRs per 100 PY for adverse events of special interest (a–i). IRs are exposure adjusted and expressed as the number of patients with events per 100 PY. IR incidence rate, MACE major adverse cardiovascular events, NMSC nonmelanoma skin cancer, PY patient-years, VTE venous thromboembolic events. Study size–adjusted IRs are per 100 PY and are shown with mid-p gamma CIs. Ritlecitinib all 30 mg includes patients who received ritlecitinib 30 mg QD with or without an initial 4-week 200-mg QD loading dose. Ritlecitinib all 50 mg and ritlecitinib 50 mg includes patients who received ritlecitinib 50 mg QD with or without an initial 4-week 200-mg QD loading dose from the placebo-controlled and all-exposure pools, respectively. aAdjudicated safety events. bIRs shown for breast cancer in female patients. cMACE was defined as a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. dVTE was defined as events of deep vein thrombosis and pulmonary embolism