Abstract
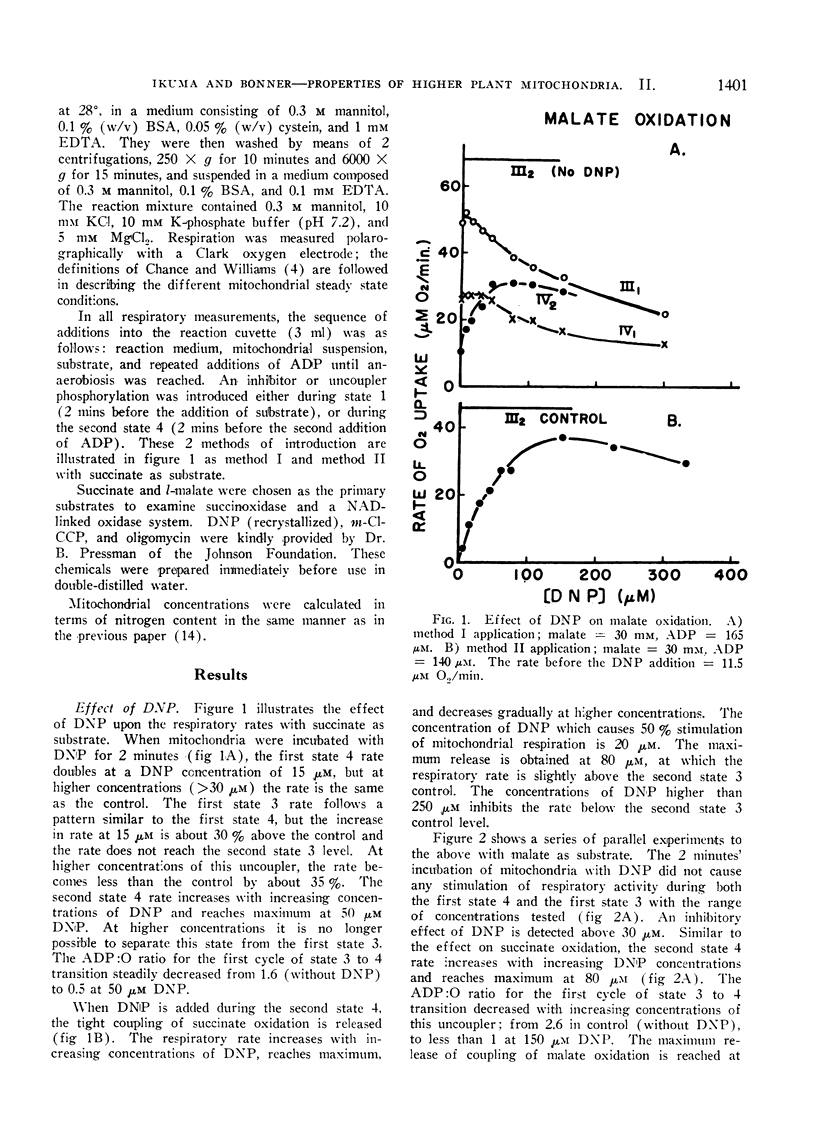
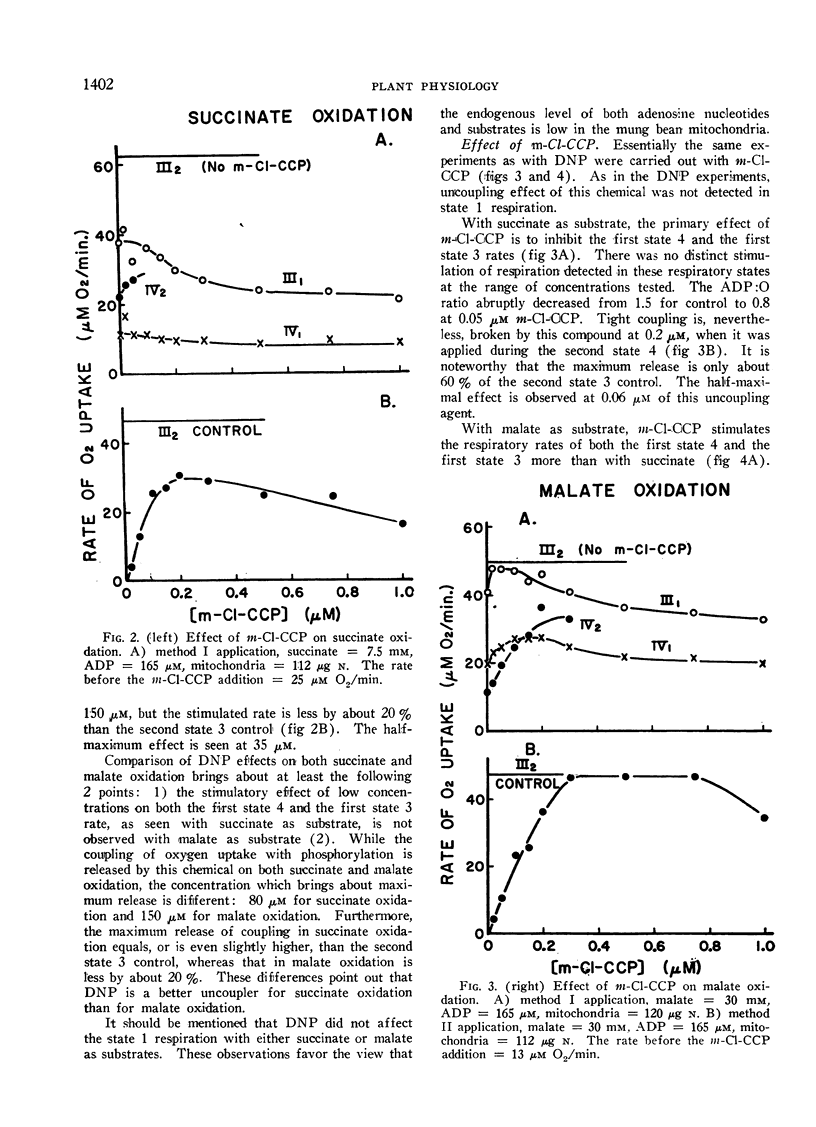
Effects of inhibitors of phosphorylation on the oxidation of succinate and of l-malate were investigated with tightly coupled mitochondria isolated from mung bean hypocotyls. When mitochondria were incubated with 2,4-dinitrophenol, or carbonyl cyanide m-chlorophenylhydrazone prior to the addition of substrate, the uncoupling effects of these chemicals were relatively small. This is probably caused by relative lack in these mitochondria of endogenous substrates, ATP, and/or “high-energy intermediates”. The action of uncoupling agents is, therefore, revealed in a more striking manner when they are introduced during the second state 4. Of the 2 uncoupling agents tested, malate oxidation consistently required 1.5 to 2 times higher concentration of the agents for the half-maximal effects than succinate oxidation. From the comparison of the degree of uncoupling it is concluded that 2,4-dinitrophenol is a better uncoupler of succinate oxidation, whereas carbonyl cyanide m-chlorophenylhydrazone functions as a more complete uncoupler of malate oxidation.
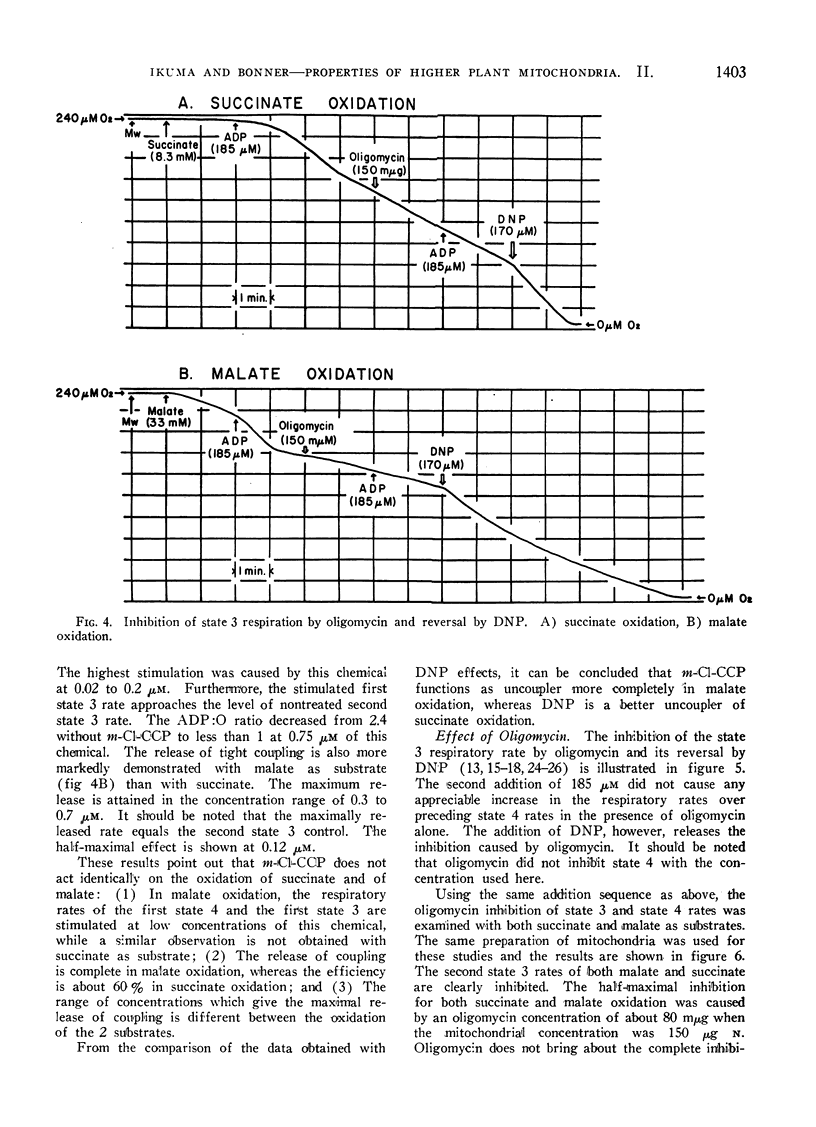
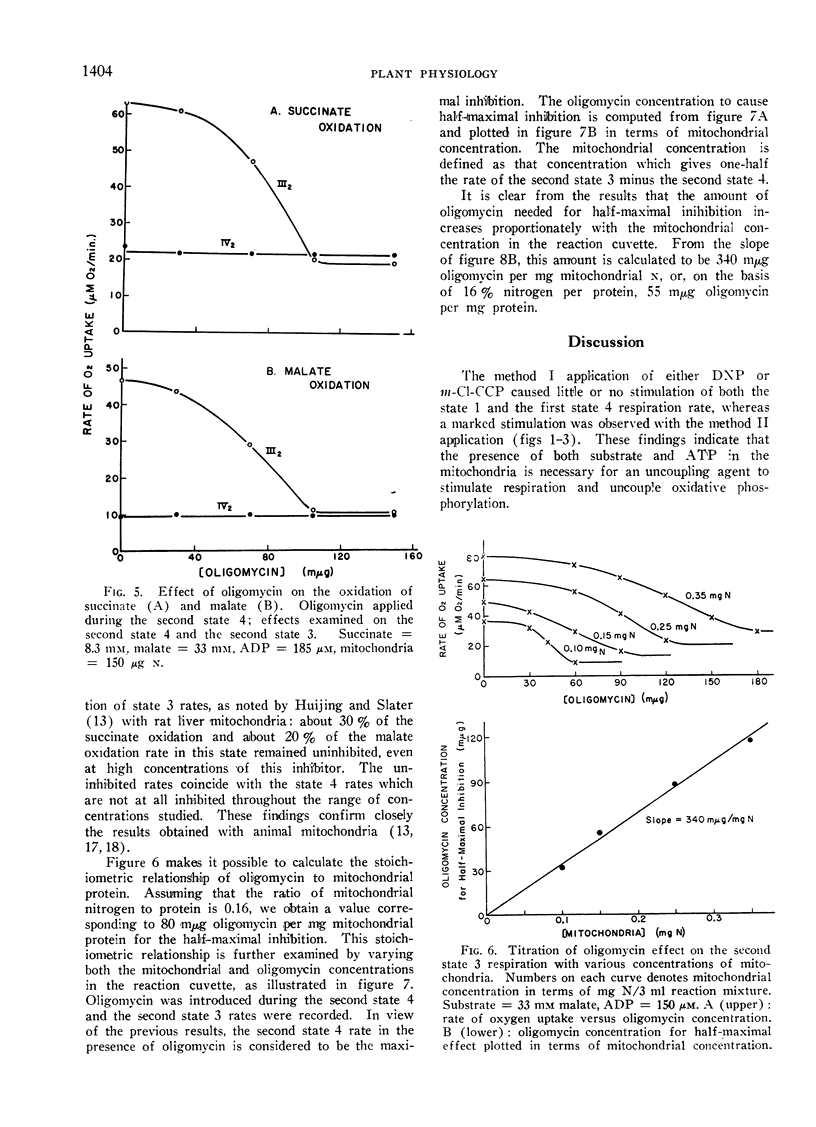
Oligomycin does not inhibit state 4 rates, while the increment of respiration due to added ADP is completely inhibited by this antibiotic. Identical half-maximal effects are observed with the same concentration of oligomycin in both succinate and l-malate oxidation. The oligomycin effect depends on the mitochondrial concentration employed. The concentration of this chemical required for the half-maximal effect is 55 to 80 mμmoles per mg mitochondrial protein. It is suggested that this inhibitor of phosphorylation binds all of the phosphorylation sites regardless of whether the sites are functional or not.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAZAWA T., BEEVERS H. Mitochondria in the endosperm of the germinating castor bean; a developmental study. Biochem J. 1957 Sep;67(1):115–118. doi: 10.1042/bj0670115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNER J., MILLERD A. Oxidative phosphorylation by plant mitochondria. Arch Biochem Biophys. 1953 Jan;42(1):135–148. doi: 10.1016/0003-9861(53)90247-1. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B. The effect of alkylguanidines on mitochondrial metabolism. J Biol Chem. 1963 Jan;238:410–417. [PubMed] [Google Scholar]
- Chappell J. B. The effects of 2,4-dinitrophenol on mitochondrial oxidations. Biochem J. 1964 Feb;90(2):237–248. doi: 10.1042/bj0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSBY R. A., HEYTLER P. G. UNCOUPLING OF OXIDATIVE PHOSPHORYLATION BY CARBONYL CYANIDE PHENYLHYDRAZONES. II. EFFECTS OF CARBONYL CYANIDE M-CHLOROPHENYLHYDRAZONE ON MITOCHONDRIAL RESPIRATION. Biochemistry. 1963 Sep-Oct;2:1142–1147. doi: 10.1021/bi00905a041. [DOI] [PubMed] [Google Scholar]
- HACKETT D. P., RICE B., SCHMID C. The partial dissociation of phosphorylation from oxidation in plant mitochondria by respiratory chain inhibitors. J Biol Chem. 1960 Jul;235:2140–2144. [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- HUIJING F., SLATER E. C. The use of oligomycin as an inhibitor of oxidative phosphorylation. J Biochem. 1961 Jun;49:493–501. doi: 10.1093/oxfordjournals.jbchem.a127334. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker V. H. Uncouplers of rat-liver mitochondrial oxidative phosphorylation. Biochem J. 1965 Dec;97(3):658–662. doi: 10.1042/bj0970658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Young R. E., Biale J. B. Metabolic Processes in Cytoplasmic Particles of the Avocado Fruit. VI. Controlled Oxidations and Coupled Phosphorylations. Plant Physiol. 1964 May;39(3):312–322. doi: 10.1104/pp.39.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]


