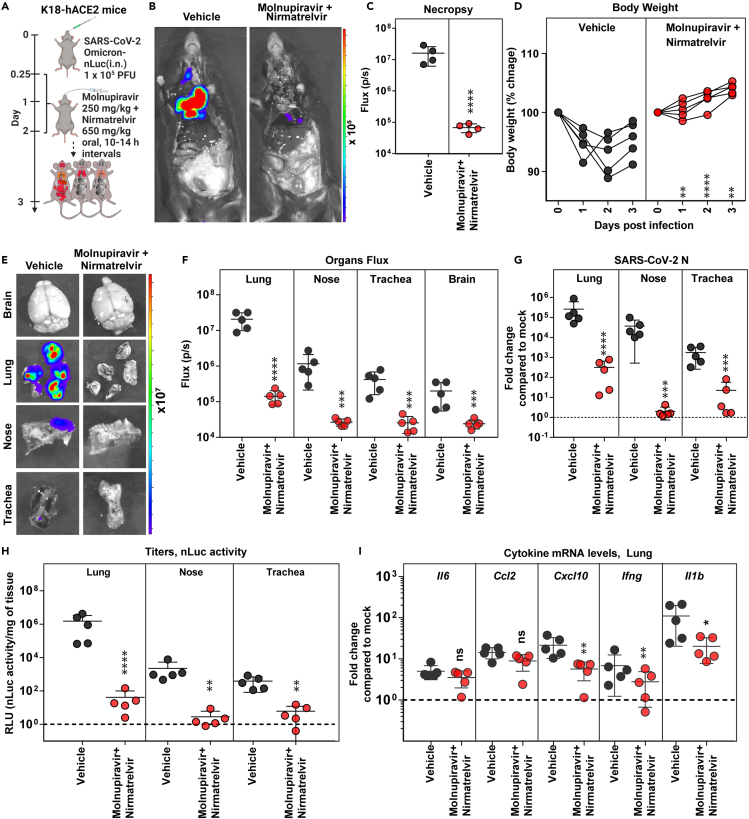
Figure 4.
Therapeutic efficacy of molnupiravir with nirmatrelvir in K18-hACE2 mice against SARS-CoV-2 Omicron VOC
(A) Experimental design for testing efficacy of molnupiravir (250 mg/kg b.w., oral) with nirmatrelvir (600 mg/kg, b.w. oral) in K18-hACE2 mice challenged with SARS-CoV-2-Omicron-nLuc (1 × 105 PFU; i.n.). The drugs were administered twice daily (BID) starting 6 hpi (0.25 dpi) for 2 days. Vehicle-treated (Vehicle) mice (n = 5) were used as control.
(B and C) Representative BLI images and temporal quantification of nLuc signal as flux (photons/sec) after necropsy at 3 dpi.
(D) Temporal changes in mouse body weight with initial body weight set to 100% for an experiment shown in A.
(E and F) Ex vivo imaging of indicated organs and quantification of nLuc signal as flux (photons/sec) after necropsy for an experiment shown in A. (G) Fold changes in nucleocapsid (N) mRNA expression in lung, nasal cavity, and trachea tissues and treatment regimens at 3 dpi. Data were normalized to Gapdh mRNA in the same sample and that in non-infected mice after necropsy.
(H) Estimated viral loads (nLuc activity/mg) from indicated tissues and treatment regimens using Vero E6 cells as targets at 3 dpi after necropsy.
(I) Fold changes in indicated cytokine mRNA expression for specified treatment regimens in the lung. Data were normalized to Gapdh mRNA in the same sample and that in non-infected mice.
Scale bars in (B) and (E) denote radiance (photons/s/cm2/steradian). The data in (C) were analyzed by t test followed by non-parametric Mann-Whitney U tests. Grouped data in (D), (F-I) were analyzed by two-way ANOVA followed by Tukey’s multiple comparison tests. ∗, p < 0.05; ∗∗, p < 0.01; ns; not significant. Mean values ±SD are depicted. b.w.: body weight.
See also Table S1.