Abstract
Uredospores of Puccinia graminis (Pers.) tritici (Eriks. and Henn.) were uniformly labeled with 14C by permitting the host (Triticum aestivum L.) to carry out photosynthesis in 14CO2 during the process of spore production by the obligate parasite. The use of 14C labeled spores provided advantages in a study of the utilization of endogenous substrates at frequent intervals with small amounts of spores under conditions conducive to germination.
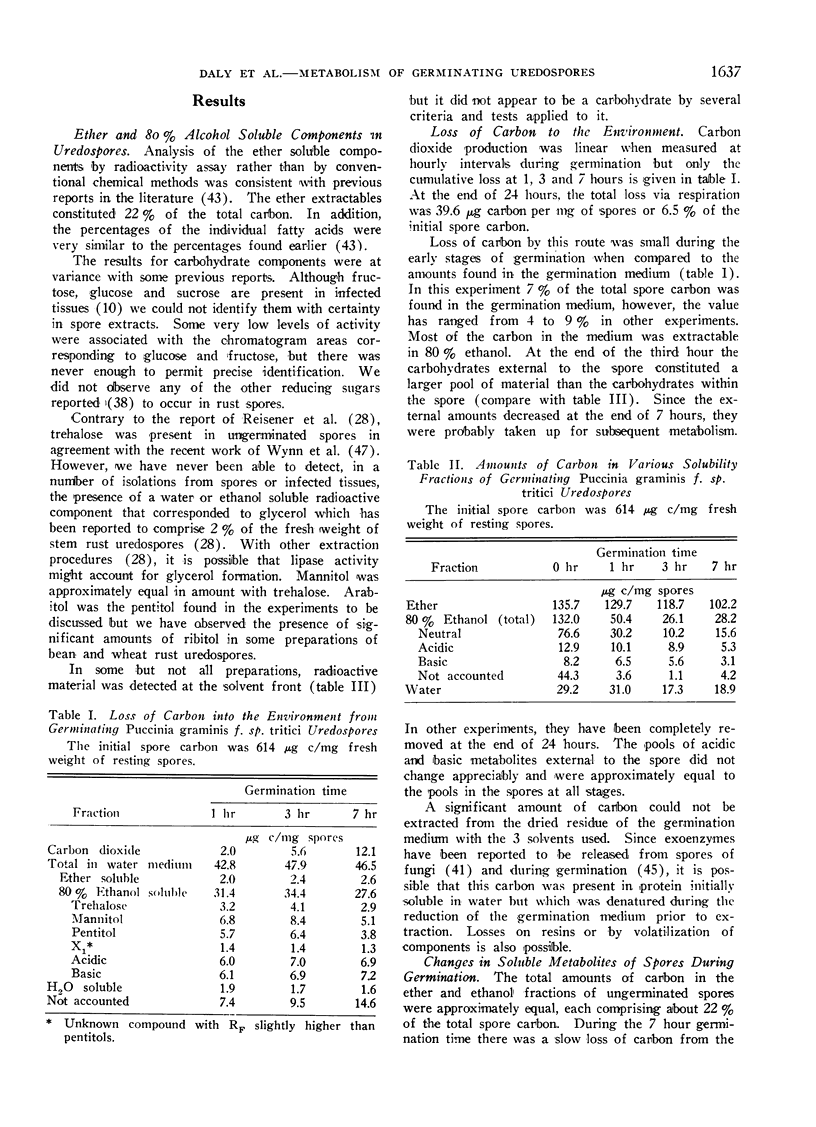
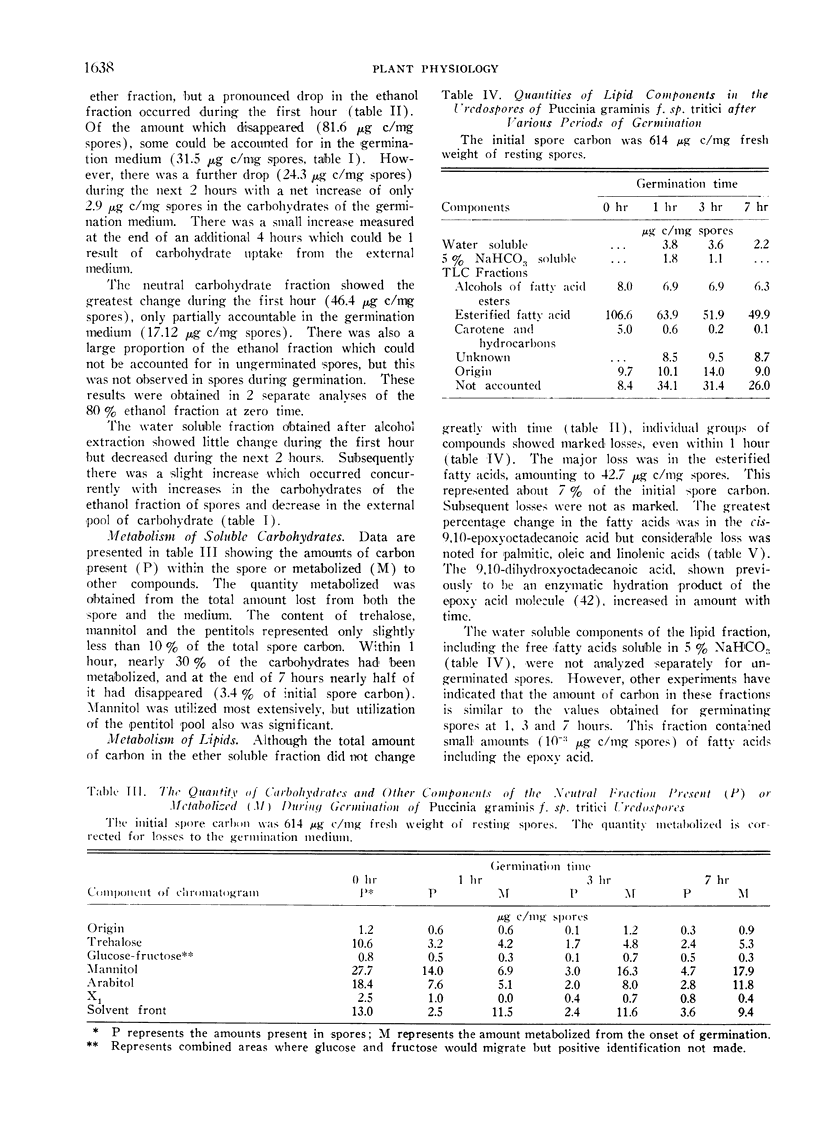
Because of previous uncertainties about the nature of the substrates of importance to germination, a detailed study of carbohydrate and lipid components, both in the spores and in the germination medium, was made during the first 7 hours after placing the spores on aqueous media. Diethyl ether and 80% ethanol soluble metabolites each constituted approximately 20% of the total spore carbon. During the first hour nearly 60% of the 80% alcohol solubles disappeared from the spores while the total ether soluble material did not change appreciably. A significant part of the 80% ethanol soluble materials appeared in the germination medium.
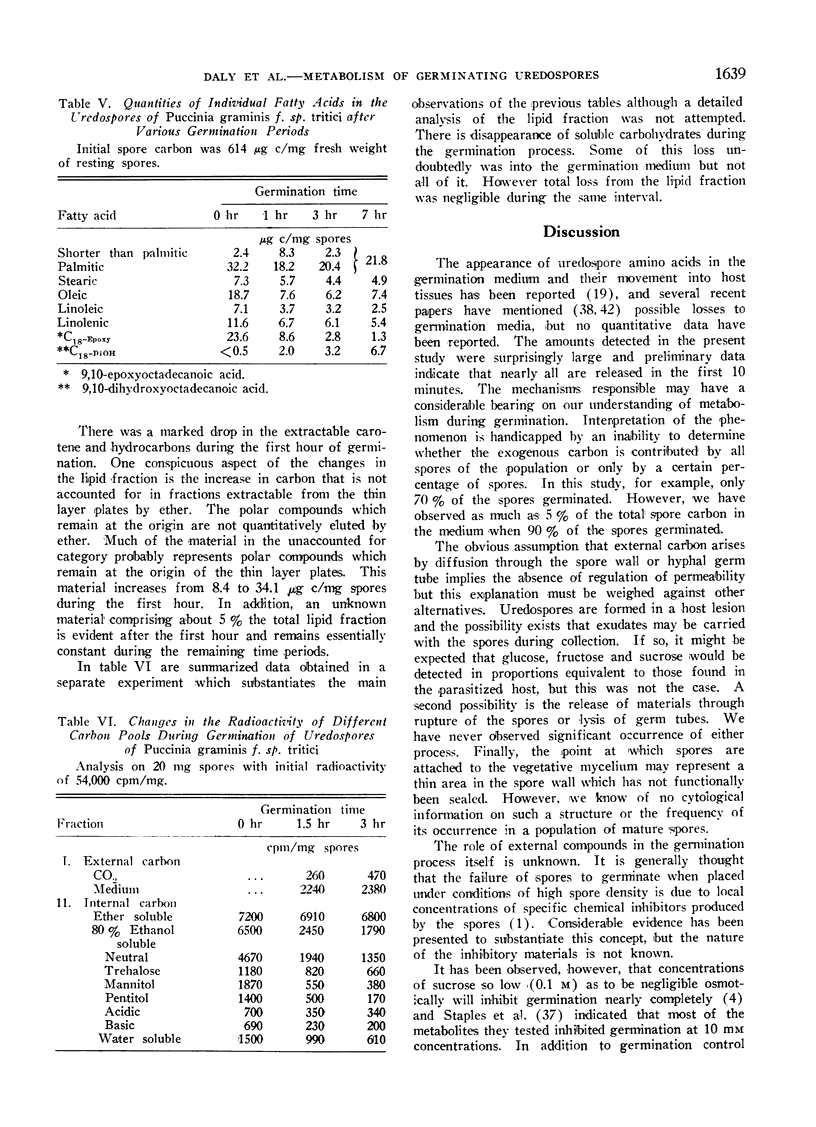
During germination and germ tube extension, there was rapid utilization of trehalose, arabitol and mannitol even though appreciable amounts of these materials were present as exogenous pools in the germination medium. Although the total amounts of ether soluble components did not change as drastically as the carbohydrate fraction, there was extensive utilization of palmitic, oleic, linolenic and 9,10-epoxyoctadecanoic acids.
The results indicate that the germination process in spores of obligate parasites is not based solely on the utilization of lipids and some possible roles of the changes in internal and external pools of soluble carbohydrates are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson T. G., Allen P. J. Purification and partial characterization of a factor in cotton wax stimulating the germination of self-inhibited wheat stem rust uredospores. Plant Physiol. 1966 Jan;41(1):28–33. doi: 10.1104/pp.41.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd K., Sussman A. S., Eilers F. I. Glucose-C14 metabolism of dormant and activated ascospores of Neurospora. J Bacteriol. 1966 Feb;91(2):551–561. doi: 10.1128/jb.91.2.551-561.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M., Deverall B. J. Metabolism of Indoleacetic Acid in Rust Diseases. I. Factors Influencing Rates of Decarboxylation. Plant Physiol. 1963 Nov;38(6):741–750. doi: 10.1104/pp.38.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M., Inman R. E., Livne A. Carbohydrate Metabolism in Higher Plant Tissues Infected With Obligate Parasites. Plant Physiol. 1962 Jul;37(4):531–538. doi: 10.1104/pp.37.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREAR D. S., JOHNSON M. A. Enzymes of the glyoxylate cycle in germinating uredospores of Melampsora lini (pers.) lev. Biochim Biophys Acta. 1961 Feb 18;47:419–421. doi: 10.1016/0006-3002(61)90312-2. [DOI] [PubMed] [Google Scholar]
- HARTMANN G. R., FREAR D. S. Enzymatic hydration of cis-9, 10-epoxyoctadecanoic acid by cell-free extracts of germinating flax rust uredospores. Biochem Biophys Res Commun. 1963 Mar 5;10:366–372. doi: 10.1016/0006-291x(63)90539-4. [DOI] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Trehalase in conidia of Aspergillus oryzae. J Bacteriol. 1966 May;91(5):1883–1887. doi: 10.1128/jb.91.5.1883-1887.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöker W., Ledingham G. A., Perlin A. S. Polysaccharides, nucleic acids, and protein of wheat stem rust (Puccinia graminis tritici) urediospores. Can J Biochem. 1965 Sep;43(9):1387–1395. doi: 10.1139/o65-156. [DOI] [PubMed] [Google Scholar]
- NIEDERPRUEM D. J., HAFIZ A., HENRY L. POLYOL METABOLISM IN THE BASIDIOMYCETE SCHIZOPHYLLUM COMMUNE. J Bacteriol. 1965 Apr;89:954–959. doi: 10.1128/jb.89.4.954-959.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISENER H. J., GOLDSCHMID H. R., LEDINGHAM G. A., PERLIN A. S. Formation of trehalose and polyols by wheat stem rust (Puccinia graminis tritici) uredospores. Can J Biochem Physiol. 1962 Sep;40:1248–1251. [PubMed] [Google Scholar]
- REISENER H., FINLAYSON A. J., MCCONNELL W. B. THE METABOLISM OF VALERATE-3-C14 AND -5-C14 BY WHEAT STEM RUST UREDOSPORES. Can J Biochem. 1964 Mar;42:327–332. doi: 10.1139/o64-038. [DOI] [PubMed] [Google Scholar]
- REISENER H., FINLAYSON A. J., McCONNELL W. B., LEDINGHAM G. A. The metabolism of propionate by wheat stem rust uredospores. Can J Biochem Physiol. 1963 Mar;41:737–743. [PubMed] [Google Scholar]
- REISENER H., McCONNELL W. B., LEDINGHAM G. A. Studies on the metabolism of valerate-1-C14 by uredospores of wheat stem rust. Can J Biochem Physiol. 1961 Oct;39:1559–1566. doi: 10.1139/o61-169. [DOI] [PubMed] [Google Scholar]
- TULLOCH A. P., LEDINGHAM G. A. The component fatty acids of oils found in spores of plant rusts and other fungi. Can J Microbiol. 1960 Aug;6:425–434. doi: 10.1139/m60-048. [DOI] [PubMed] [Google Scholar]
- TUREL F. L., LEDINGHAM G. A. Utilization of labelled substrates by the mycelium and uredospores of flax rust. Can J Microbiol. 1959 Oct;5:537–545. doi: 10.1139/m59-064. [DOI] [PubMed] [Google Scholar]
- VAN SUMERE C. F., VAN SUMERE-DE, PRETER C., LEDINGHAM G. A. Cell-wall-splitting enzymes of Puccinia graminis var. tritici. Can J Microbiol. 1957 Aug;3(5):761–770. doi: 10.1139/m57-086. [DOI] [PubMed] [Google Scholar]


