Fig. 2. Spatio-temporal behavior of corneal cells encapsulated within s-HA hydrogels.
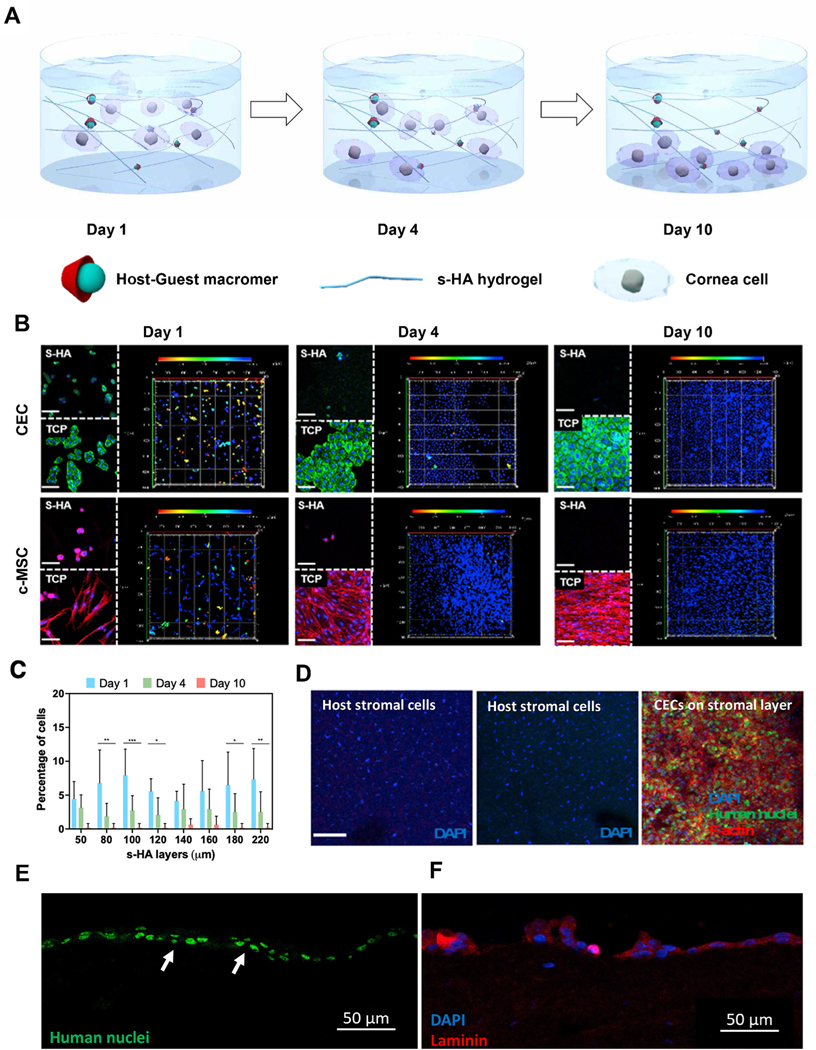
(A) Schematic illustration for the behavior of encapsulated corneal cells at days 1, 4, and 10. At day 0 most cornea cells are present throughout s-HA hydrogel layers; at day 4, some cells adhere to the TCP while others continue to be present through the hydrogel layers; at day 10, all cells adhere and spread on the TCP. (B) Confocal images of encapsulated cornea cells in s-HA hydrogel stained with phalloidin exhibiting F-actin on the TCP and within the hydrogel at days 1, 4, and 10 (green for CECs; red for c-MSCs; blue for DAPI, scale bar: 100 μm). The 3D images show the depth of the cornea cells throughout the hydrogel. The different colors represent the layers where the cells belong; red - 0 μm for the most outer layer and blue - 220 μm for the 2D TCP. (C) Quantification of the cell distribution within the s-HA hydrogel at days 1, 4, and 10 (n = 8); the percentage of cells distributed within the same layer as day 1 statistically decreases at day 4 (***p < 0.0001, **p < 0.001, and * p = 0.03) and at day 10 few cells are observed within the hydrogel (D) Confocal images show that encapsulated CEC in s-HA (green) adhere to the stromal layer of ex vivo rabbit cornea after 4 days (3), whereas no CECs encapsulated in linear HA is able to adhere to the cornea (2) and was similar to the no treatment group (1). CECs are stained with human nuclei cy3 (green), phalloidin (red), and DAPI (blue). DAPI staining shows stromal nuclei of host stromal layer and delivered CEC (scale bar: 100 μm). Immunostaining of section of the corneas shows that CECs staining with (E) anti-human nuclei cy3 (green) are able to form a double layer and (F) laminin (red) at day 4.
