Abstract
Objectives
Chronic obstructive pulmonary disease (COPD) disproportionately affects low- and middle-income countries. Health systems are ill prepared to manage the increase in COPD cases.
Methods
We performed a pilot effectiveness-implementation randomized field trial of a community health worker (CHW)-supported, 1-year self-management intervention in individuals with COPD grades B–D. The study took place in low-resource settings of Nepal, Peru, and Uganda. The primary outcome was the St. George’s Respiratory Questionnaire (SGRQ) score at 1 year. We evaluated differences in moderate to severe exacerbations, all-cause hospitalizations, and the EuroQol score (EQ-5D-3 L) at 12 months.
Measurements and Main Results
We randomly assigned 239 participants (119 control arm, 120 intervention arm) with grades B–D COPD to a multicomponent, CHW-supported intervention or standard of care and COPD education. Twenty-five participants (21%) died or were lost to follow-up in the control arm compared with 11 (9%) in the intervention arm. At 12 months, there was no difference in mean total SGRQ score between the intervention and control arms (34.7 vs. 34.0 points; adjusted mean difference, 1.0; 95% confidence interval, −4.2, 6.1; P = 0.71). The intervention arm had a higher proportion of hospitalizations than the control arm (10% vs. 5.2%; adjusted odds ratio, 2.2; 95% confidence interval, 0.8, 7.5; P = 0.15) at 12 months.
Conclusions
A CHW-based intervention to support self-management of acute exacerbations of COPD in three resource-poor settings did not result in differences in SGRQ scores at 1 year. Fidelity was high, and intervention engagement was moderate. Although these results cannot differentiate between a failed intervention or implementation, they nonetheless suggest that we need to revisit our strategy.
Clinical trial registered with www.clinicaltrials.gov (NCT03359915).
Keywords: COPD, self-management, global health, community health workers
At a Glance Commentary
Scientific Knowledge on the Subject
COPD is projected to become the third leading cause of death by 2030. A significant proportion of COPD cases in low- and middle-income countries (LMICs) remains undiagnosed and untreated due to several challenges including a lack of health system resources and capacity. Guideline-based interventions for COPD, such as action plans, have been demonstrated to improve COPD outcomes in high-income settings. In addition, task-shifting strategies, such as community health worker models, have shown success in LMICs in addressing chronic diseases such as hypertension and diabetes. Community health worker (CHW) models may also represent effective models for delivering evidence-based care for chronic respiratory diseases.
What This Study Adds to the Field
In this pilot effectiveness-implementation trial of adults with moderate-to-severe COPD, we assessed the feasibility of testing a CHW-supported self-management intervention for COPD on respiratory-related quality of life, moderate-to-severe exacerbations, and all-cause hospitalizations. We carried out this intervention across three low-resource community settings in three continents that varied in culture, urbanization, and healthcare resources. We did not observe significant improvements in effectiveness outcomes. However, this pilot trial provides insight into the important considerations for implementing COPD self-management interventions in such contexts, where availability and access to evidence-based care is limited.
Chronic obstructive pulmonary disease (COPD) is a progressive, life-threatening lung disease that was estimated to be responsible for 3.2 million deaths worldwide in 2019. COPD is projected to become the third leading cause of death by 2030, and more than 90% of COPD deaths occur in low- and middle-income countries (LMICs) (1). Because of a lack of resources and capacity, health systems in these countries are ill prepared to diagnose, treat, and manage the growing burden of COPD. Indeed, a significant proportion of COPD cases in LMICs remain undiagnosed and untreated (2, 3).
Effective treatments and self-management interventions for COPD, such as action plans, have been demonstrated to reduce symptoms, prevent exacerbations, and improve health-related quality of life in high-income settings (4). Self-management interventions comprise multicomponent behavioral strategies that allow individuals to play a central role in managing their own health behaviors and treatments, often in collaboration with caregivers or other support in their social networks (5). COPD action plans serve as tools to guide individuals to monitor and recognize changes in symptoms and use appropriate, evidence-based therapies and healthcare-seeking behaviors to manage COPD exacerbations (6).
In LMICs, task-shifting strategies have been used to address the limited resources for training of healthcare workers (7). Community health worker (CHW) models have shown success in allowing the health system to provide care more directly and effectively to communities, including in LMICs. These models show promise for diseases requiring chronic care, such as hypertension and diabetes (8, 9). Because of their potential for scalability and leveraging of existing infrastructure and personnel from ongoing programs, CHW models may also represent effective models for delivering evidence-based, self-management–based care for respiratory diseases such as COPD. Multicomponent, CHW-based strategies for obstructive lung diseases have shown mixed results for improving quality of life, respiratory admissions, and mortality in high-income settings (10). However, these task-shifting models may be particularly useful in LMIC settings, where existing infrastructure for COPD treatment and management is limited. In this pilot effectiveness-implementation trial of adults with moderate to severe COPD, we sought to assess the feasibility of testing a multicomponent, CHW-supported self-management intervention for COPD on respiratory-related quality of life (11), health-related quality of life, moderate to severe exacerbations, and all-cause hospitalizations over 12 months.
Methods
Study Setting
The protocol for the GECo2 (Global Excellence in COPD outcomes) trial was published previously (12), and the trial was registered with www.clinicaltrials.gov (NCT03359915). Briefly, the trial took place in three geographically, economically, and culturally diverse regions in Asia, South America, and sub-Saharan Africa (Table 1). Sites were selected to test the performance of a CHW-supported action plan for COPD management in different continents and at sites with different degrees of urbanization, prevalence of COPD, and economic development. Trial participants were recruited from a random, age- and sex-stratified sample of adults aged 40–95 years who underwent spirometry (13) as part of an earlier study reporting the discriminative accuracy of COPD screening instruments in the same three settings (14).
Table 1.
Economic and Demographic Characteristics of Study Sites
| Nepal | Peru | Uganda | |
|---|---|---|---|
| Classification by income level | Low income | Upper middle income | Low income |
| Country region | South Asia | South America | East Africa |
| Country population (2018) | 26.5 million | 32.2 million | 41.5 million |
| Rural population, % | 80% | 22% | 76% |
| Gross domestic product | $33 billion USD | $223 billion USD | $40.5 billion USD |
| Percentage living below the poverty line (2019) | 15% | 20% | 21% (2016) |
| Study site (urbanization status) | Bhaktapur (periurban) | San Juan de Miraflores, Lima (urban) | Nakaseke (rural) |
Data from Reference 30.
Study Design
The GECo2 study was a single-blind, individually randomized controlled pilot trial performed between March 9, 2018, and July 17, 2020. Before testing the multicomponent intervention, we performed 8 months of formative research consisting of qualitative interviews with individuals with COPD, health providers, and CHWs to adapt COPD education material and the COPD action plan for patients, allow instrument development, gain an understanding of pathways to care for COPD, and tailor CHW training. Formative research informed content and adaptation of the COPD education materials and action plan (instruments found in the protocol paper) (12, 15).
We consecutively approached and enrolled eligible individuals from an age- and sex-stratified, population-based sample of adults aged ⩾40 years who underwent spirometry testing (13) and were identified to have COPD by post-bronchodilator spirometry with severity grades B–D (16). Participants were considered to have COPD if they had a post-bronchodilator FEV1/FVC below the lower fifth percentile of the 2012 Global Lung Function Initiative mixed ethnic population for their given age, sex, and height (i.e., an FEV1/FVC z-score less than −1.645) and grades B–D spirometry based on 2017 Global Initiative for Chronic Obstructive Lung Disease guidelines (16). Exclusion criteria were self-reported pregnancy, self-reported active pulmonary tuberculosis, or receiving medications for pulmonary tuberculosis, or contraindications to spirometry (eye surgery, thoracic surgery, abdominal surgery, or myocardial infarction in the 3 months before the study visit or measured blood pressure >180/100 mm Hg at the research assessment). Participants were randomly selected from study area censuses, regardless of respiratory symptoms, a prior diagnosis of COPD, St. George’s Respiratory Questionnaire (SGRQ) scores, or exacerbation history at baseline. We made a pragmatic choice not to require guideline-recommended maintenance therapy because of the extremely low availability and affordability of these therapies in these settings (17).
We aimed to randomize 240 adults (80 in each country) with COPD who met eligibility criteria over 12 months and see them in follow-up quarterly for 1 year to evaluate primary and secondary outcomes. Participants were randomized 1:1 to either the intervention or control arm using an online system (18), stratified by country. We used randomly permuted block sizes of between 2 and 6. Principal investigators and data analysts were blinded to treatment allocation. Because of the nature of the intervention, it was not feasible to blind participants or data collectors to treatment assignment. Although we planned to enroll 80 participants from each site, we found that the prevalence of COPD in Lima, Peru, was lower than originally anticipated in the parent study (14). Given that enrollment was done consecutively and across study settings, once we realized that our site in Lima had fewer eligible grades B–D COPD cases, we increased the number of participants enrolled in Uganda and Nepal to meet our recruitment targets and timeline.
Intervention and Control Conditions
Individuals were randomized to receive either a CHW-based self-management intervention or usual care with COPD education. The intervention consisted of four components surrounding prevention and self-management of COPD and monthly CHW visits over 1 year. Selection of intervention components was guided by the Capability, Opportunity, and Motivation of Behavior framework (19) and based on formative research related to key barriers to and facilitators of adopting COPD self-management practices (12, 15). These components included COPD education at enrollment; training and ongoing support in self-management of acute exacerbations using a context-adapted action plan, which included training and support on recognition of symptoms; rescue packs delivered or refilled by a CHW consisting of antibiotics and steroids for use during exacerbations; and continuous and iterative reinforcement and feedback on COPD educational concepts and self-management behaviors, such as smoking cessation and home-based exercise. Rescue packs consisted of 30 mg of prednisolone taken once daily for 5 days and 500 mg of amoxicillin taken three times per day for 5 days. If amoxicillin was not available or the participant was allergic to penicillin, it was replaced with either 500 mg of azithromycin taken daily for 3 days (Peru and Uganda) or 200 mg of doxycycline on the first day followed by 100 mg for the remaining 4 days (Nepal). CHWs also served as a source of support for navigating the healthcare system. In Peru, eight intervention participants received their final monthly CHW visits via telephone during the months of March through June 2020 because of the COVID-19 pandemic.
Participants randomly assigned to the control arm received basic COPD education from a CHW and were offered access to the same medications for acute exacerbations free of charge at designated local clinics or pharmacies. The study teams in each country ensured that the medications were available at these designated locations.
CHW Training and Rollout
CHWs at each site were recruited from local catchment areas and trained in the delivery of the COPD education tool, the COPD action plan, use of rescue packs, referral to higher levels of care, provision of patient navigation services, and longitudinal reinforcement of COPD education concepts and self-management behaviors. They were also trained in the distribution of antibiotics and steroids during home visits, building patient rapport, and effective communication. The training included a combination of didactic instruction and role-playing activities over the course of 2 weeks with regular feedback. We conducted refresher training as needed and after any protocol adaptations (see the online supplement). A total of 8 CHWs were recruited in Nepal, 3 in Peru, and 13 in Uganda.
Standardization and Assessment of Fidelity to the Intervention Protocol
We assessed fidelity to the intervention using direct observations of CHW visits and fidelity checklists (online supplement). Field team supervisors completed fidelity checks to observe key standardized competencies of CHWs and adherence to the study protocol during home visits. We observed CHWs three times: at an initial participant visit, at a 4–6-month visit, and at a 10–12-month visit.
To standardize the content of the CHW visits, we created a standard training and retraining protocol across all three sites (online supplement). CHWs met with site leaders weekly to discuss any issues that arose and to provide an opportunity for retraining as needed on the basis of fidelity observation visits.
Study Outcomes
The primary outcome for this trial was respiratory-related quality of life, defined as the SGRQ total score at 12 months (11). The SGRQ has previously been validated in Spanish (20), and our research team previously conducted validation studies of the SGRQ in both Luganda and Nepali (21, 22). We also examined differences between treatment arms in SGRQ score at earlier follow-up visits. Secondary outcomes included the proportions of moderate to severe exacerbations and all-cause hospitalizations over 12 months, SGRQ subscores, the five-dimension, three-level EuroQol health-related quality life scale (EQ-5D-3 L) at 12 months, and the EQ-5D visual analog scale score (23). Moderate exacerbations were defined as having taken a rescue pack in the follow-up period without hospitalization. Severe exacerbations were defined as having been hospitalized for COPD during the follow-up period.
Study Procedures
Participants were visited in person at their homes by independent data collectors at baseline and quarterly thereafter over 1 year for a total of five visits. We evaluated engagement with intervention components and fidelity to the intervention using a mixed-methods approach. First, we measured indicators of engagement with the intervention, including use of the action plan by participants and rescue pack use, at each of the quarterly follow-up visits via questionnaire. We also performed semistructured interviews with 17 participants (5 in Nepal, 5 in Peru, 7 in Uganda) and 11 CHWs (2 in Nepal, 2 in Peru, 7 in Uganda) during the follow-up period and with three field supervisors (one in Nepal, one in Peru, and one in Uganda). Each interview lasted between 30 and 60 minutes. Interviews with participants were performed in the local language and translated into English by qualified translators for analyses. CHWs completed visit logs after each monthly visit. During the initial and monthly home visits, CHWs recorded notes about their interactions with participants, including whether the participant was receptive to the visit, topics discussed during the education session, and observations regarding participant use of the action plans and rescue packs.
Biostatistical Methods
We took the approach recommended by Cocks and Torgerson (24) for sample size calculation of a pilot trial. We calculated that 112 participants per arm would be needed to produce an 80% one-sided confidence interval that excluded a 4-point difference in total SGRQ score and an SD of 25 points under the scenario of no difference in means (25). A final sample size of 240 participants would thus allow greater precision while accounting for a 5–10% loss to follow-up. We conducted analyses in R version 4.2.2 (26) on the basis of a predefined statistical analysis plan.
All analyses were performed on an intention-to-treat basis. We compared the total SGRQ score at 12 months between trial arms using a linear regression model adjusted for baseline SGRQ score and study site. We conducted a similar analysis for SGRQ subscores. In sensitivity analyses, we adjusted for age, sex, the modified Medical Research Council dyspnea scale score, and prebronchodilator FEV1 (in liters). In a sensitivity analysis that included data for all 239 participants, we estimated mean differences in total SGRQ scores between the intervention and control arms at 3, 6, 9, and 12 months after randomization using a linear mixed effects regression model, which included an evaluation of intervention arm by time-point interactions and adjusted for site and a random intercept by subject.
In secondary analyses, we compared the proportions of participants who experienced all-cause hospitalization and moderate to severe COPD exacerbations at 12 months between study arms using a log-binomial regression to estimate relative risk. We used linear regression to estimate differences in mean EQ-5D-3 L scores at 12 months adjusted for baseline scores and study. Missing data were assumed to be missing at random. We therefore used complete case analyses.
Qualitative Data Analysis
In-depth interviews were digitally recorded, transcribed, and translated into English by bilingual professionals as needed. Handwritten CHW observation notes from each home visit were translated into English for coding purposes. We developed a codebook that included several thematic codes relevant to engagement with the intervention components and fidelity through a process of initial line-by-line coding followed by group discussion and consensus. We then used the codebook to interpret the interview transcripts and field notes and identified quotations relevant to engagement with the action plan.
Ethical Considerations
This trial was approved by the ethics review boards of Johns Hopkins University School of Medicine (IRB00139901) in Baltimore, Maryland; University College London (9661/001) in London, United Kingdom; PRISMA Charitable Association in Lima, Peru (CE2147.17); Makerere University in Kampala, Uganda (REC 2017-096); and the Nepal Health Research Council (Reg. No. 136/2017) in Kathmandu, Nepal. All participants provided written informed consent.
Role of Funding Source
The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Results
Participant Characteristics
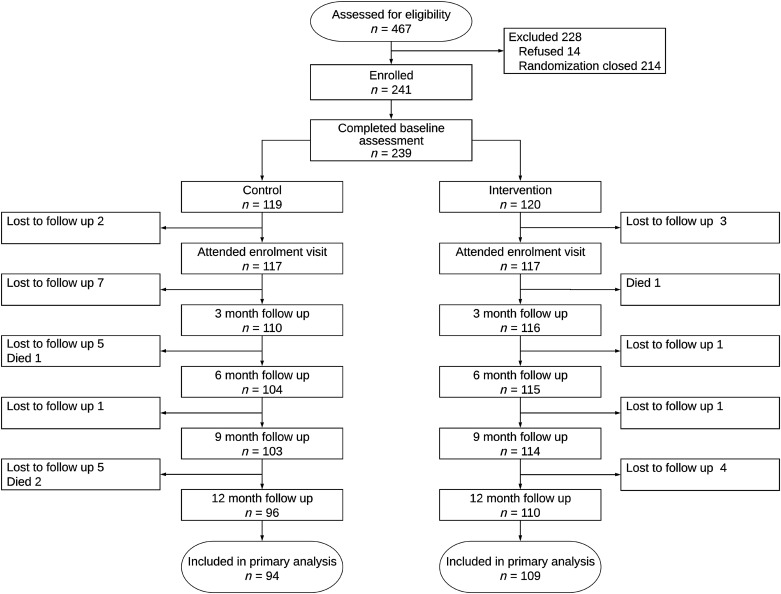
A total of 10,664 participants (3,534 in Nepal, 3,550 in Peru, and 3,580 in Uganda) participated in the parent study, and 467 (147 Nepal, 73 Peru, and 247 Uganda) were identified as having grades B–D COPD (14). Of these, we consecutively enrolled the first 241 participants who agreed to participate in the trial. Two participants were later found not to have grades B–D COPD. We therefore randomized 239 participants; 120 were assigned to the intervention arm and 119 to the control arm (Figure 1). Intervention participants were, on average, 3 years older than those in the control arm but otherwise had similar characteristics (Table 2). Intervention participants had similar lung function at baseline when compared with control participants and low use of COPD medications at baseline. A total of 33 participants were lost to follow-up (20 in the control arm and 9 in the intervention arm), and 4 died (3 in the control arm and 1 in the intervention arm) over the 12 months of follow-up (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram of participant flow through the GECo2 study.
Table 2.
Baseline Characteristics of Participants in Intervention and Control Arms
| Characteristic | Intervention | Control |
|---|---|---|
| Age, yr, mean (SD) | 68.0 (10.9) | 65.1 (10.8) |
| Number of females (%) | 52 (43.3) | 45 (37.8) |
| Income in USD/mo, mean (SD) | 116.8 (156.8) | 133.8 (184.6) |
| Number of current smokers (%) | 29 (24.2) | 25 (21.0) |
| Previous diagnosis of pulmonary tuberculosis (%) | 13 (10.8) | 16 (13.4) |
| Uses biomass daily to cook, n (%) | 51 (42.5) | 52 (43.7) |
| Body mass index, kg/m2, mean (SD) | 22.5 (4.3) | 22.9 (5.0) |
| Lung function | ||
| Post-bronchodilator FEV1 z-score, L, mean (SD) | −2.08 (1.23) | −2.19 (1.16) |
| Post-bronchodilator FEV1 percentage predicted, mean (SD) | 64.5% (21.5%) | 63.5% (20.2%) |
| Post-bronchodilator FEV1/FVC z-score, mean (SD) | −2.87 (0.95) | −2.94 (1.04) |
| Post-bronchodilator FEV1/FVC, mean (SD) | 0.56 (0.10) | 0.56 (0.11) |
| COPD category, n (%) | ||
| B | 79 (66.4) | 97 (80.8) |
| C | 3 (2.5) | 3 (2.5) |
| D | 31 (26.1) | 17 (14.2) |
| Site, n (%) | ||
| Nepal | 49 (41.2) | 51 (42.5) |
| Peru | 20 (16.8) | 20 (16.7) |
| Uganda | 50 (42.0) | 49 (40.8) |
| Prior chronic respiratory disease diagnosis, n (%) | ||
| COPD | 10 (8.3) | 10 (8.4) |
| Chronic bronchitis | 40 (33.3) | 39 (32.8) |
| Emphysema | 1 (0.8) | 1 (0.8) |
| Comorbidities, n (%) | ||
| Hypertension | 32 (26.7) | 23 (19.3) |
| Heart disease | 6 (5.0) | 4 (3.4) |
| Angina | 4 (3.3) | 1 (0.8) |
| Diabetes | 8 (6.7) | 7 (5.9) |
| Lung cancer | 0 (0) | 0 (0) |
| Tuberculosis | 13 (10.8) | 16 (13.4) |
| Regular medication use, n (%) | ||
| Inhaled corticosteroids | 1 (0.8) | 2 (1.7) |
| Short-acting β-agonists | 9 (7.5) | 8 (6.7) |
| Short-acting antimuscarinic | 3 (2.5) | 6 (5.0) |
| Long-acting β-agonists | 5 (4.2) | 4 (3.4) |
| Long-acting antimuscarinic | 4 (3.3) | 4 (3.4) |
| Xanthines | 0 (0.0) | 1 (0.8) |
| Noninhaled steroids | 1 (0.8) | 2 (1.7) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Difference in Total SGRQ Score
There were no differences in total SGRQ score between the intervention and control arms at 12 months (Table 3) or at any quarterly visit (Figure 2). After adjusting for total SGRQ score at baseline and study site, the difference remained small (mean difference, 1.0; 95% confidence interval, −4.2 to 6.1; P = 0.71). In a sensitivity analysis that included all participants (120 intervention and 119 control participants) and used all SGRQ scores collected between 3 and 12 months, the difference in total mean SGRQ scores between the intervention and control arms was 0.2 (95% confidence interval, −4.3 to 4.6). We did not identify an interaction between the intervention arm and time point in this multiple time-point analysis (P = 0.54).
Table 3.
Unadjusted and Adjusted Differences in Primary and Secondary Outcomes, by Study Arm at 12-Month Follow-Up
| Outcome | Intervention (n = 110) | Control (n = 96) | Mean Unadjusted Difference (95% CI) or RR (95% CI) | Mean Adjusted Difference (95% CI) or RR (95% CI) |
|---|---|---|---|---|
| Total SGRQ score, mean (SD)* | 34.7 (20.2) | 34.0 (20.8) | 0.6 (−5.1 to 6.3) | 1.0 (−4.2 to 6.1) |
| SGRQ subscores* | ||||
| Impact, mean (SD) | 26.2 (21.6) | 27.8 (22.6) | −1.6 (−7.8 to 4.5) | −1.0 (−6.5 to 4.5) |
| Activity, mean (SD) | 50.6 (25.4) | 45.3 (24.7) | 5.3 (−1.6 to 12.3) | 5.2 (−1.1 to 11.4) |
| Symptoms, mean (SD) | 32.3 (18.0) | 32.9 (24.1) | −0.6 (−6.6 to 5.4) | −0.2 (−5.7 to 5.3) |
| Participants experiencing at least one hospitalization, n (%) | 11 (10.0) | 5 (5.2) | 1.9 (0.7 to 5.2) | 2.2 (0.8 to 7.5) |
| Participants receiving treatment for at least one moderate-to-severe exacerbation, n (%) | 78 (70.9) | 26 (27.1) | 1.4 (0.8 to 1.9) | 3.0 (0.7 to 2.1) |
| EQ-5D-3 L score, mean (SD) | 7.5 (1.8) | 7.8 (2.2) | −0.03 (−0.9 to 0.3) | −0.02 (−0.7 to 0.3) |
| EQ-5D visual analog scale score, mean (SD) | 69.1 (14.8) | 71.3 (15.1) | −2.1 (−6.2 to 2.1) | −1.7 (−5.4 to 2.1) |
Definition of abbreviations: CI = confidence interval; EQ-5D-3 L = five-dimension, three-level EuroQol health-related quality of life questionnaire; RR = relative risk; SGRQ = St. George’s Respiratory Questionnaire.
These outcomes include the SGRQ scores (adjusted for site and baseline value), the percentages of participants who experienced hospitalizations or moderate to severe exacerbations (adjusted for site), and the EQ-5D-3 L and EQ-5D visual analog scale scores (adjusted for site and baseline values).
SGRQ scores at 12 months were missing in three participants (one intervention arm, two control arm).
Figure 2.
Comparison of differences in SGRQ total score and subscores (activity, impacts, symptoms) at baseline and 3-, 6-, 9-, and 12-month follow-up visits between the intervention and control arms. The blue lines represent the intervention arm, and the red lines represent the control arm. The diamond point estimates indicate the means, the thicker lines represent the 80% one-sided confidence intervals, and the thinner lines represent the 95% confidence intervals. SGRQ = St. George’s Respiratory Questionnaire.
Differences in Secondary Outcomes
There were no differences in SGRQ subscores between the intervention and control arms at 12 months (Table 3) or at any other quarterly visit (Figure 2). Models adjusting for age, sex, and disease severity and sensitivity analyses adjusting for predictors of missingness gave similar results. There were also no differences in EQ-5D-3 L scores or in the EQ-5D visual analog scale score at 12 months (Table 3). At 12 months, intervention participants had a higher proportion of hospitalizations and moderate to severe exacerbations for which they received treatment than control participants (Table 3).
Indicators of Engagement with the Intervention
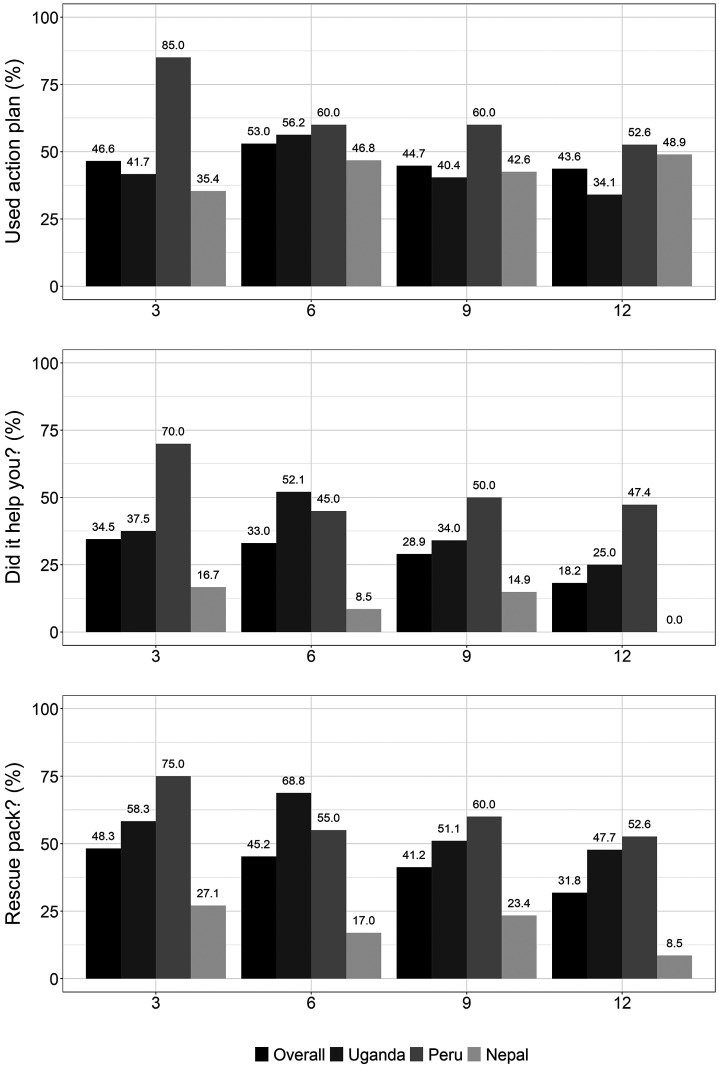
The overall percentages of individuals in the intervention arm who reported using their action plans at each follow-up time point (3, 6, 9, and 12 mo) were 46.6%, 53.0%, 44.7%, and 43.6%, respectively (Figure 3). We also show the mean (SD) number of rescue packs used by the intervention arm in Table 4. Field notes were consistent with survey results in that few participants across sites referred directly to the COPD action plan for their COPD management. Some participants reported memorizing the action plan contents instead of consulting the plan directly. Others used them only for the pulmonary rehabilitation exercises, whereas some did not use them at all.
Figure 3.
Indicators of engagement with the intervention among intervention arm participants. The top panel displays the percentage of participants, overall and in each site, at 3-, 6-, 9-, and 12-month follow-up visits, who answered “yes” to the question, “During the last 3 months, have you used your action plan?” The second panel displays the percentage of participants who answered “yes” to the question, “[Among those who did use the action plan], did your action plan help guide your decision to take medications or seek medical care?” The third panel displays the percentage of participants who answered “yes” to the question, “During the last 3 months, did you use a rescue pack?”
Table 4.
Mean (SD) Number of Rescue Packs Used over 3 Months, by Intervention Arm and Time Point
| Mean (SD) Number of Rescue Packs Used in 3-Mo Intervals |
||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention |
Control |
|||||||
| Overall | Uganda | Peru | Nepal | Overall | Uganda | Peru | Nepal | |
| 3 mo | 1.1 (1.6) | 1.4 (1.9) | 1.2 (1.1) | 0.7 (1.4) | 0.2 (0.8) | 0.4 (1.1) | 0.2 (0.6) | 0.0 (0.0) |
| 6 mo | 1.1 (1.7) | 1.5 (1.8) | 1.4 (1.7) | 0.5 (1.4) | 0.2 (0.6) | 0.5 (0.9) | 0.1 (0.3) | 0.0 (0.0) |
| 9 mo | 0.7 (1.2) | 1.0 (1.4) | 1.2 (1.2) | 0.2 (0.4) | 0.3 (0.9) | 0.8 (1.2) | 0.0 (0.0) | 0.0 (0.0) |
| 12 mo | 0.6 (1.1) | 0.9 (1.2) | 0.8 (1.0) | 0.2 (0.8) | 0.2 (0.5) | 0.4 (0.8) | 0.0 (0.0) | 0.0 (0.0) |
“I know everything from the Action Plan booklet by reading it many times, that’s why I don’t read it anymore.” (field notes, Nepal).
There was also evidence that understanding of the action plan zones did not always align with what the intervention was intended to communicate.
“When I’m in the yellow zone…, it’s because I’m improving, right?… So, if I don’t improve, I’d be in the red zone, the red zone is danger, right? So now, I’ve been taking my pills and all, I’m improving, I’m in the amber [yellow] zone, and I want to get to the green zone.… I hope I get there. (participant, Peru)
Finally, low literacy in Nepal was cited as a barrier to use of the action plan.
The percentages of intervention participants who reported using rescue packs during the previous 3 months were 48.3%, 45.2%, 41.2%, and 31.8%, respectively (3, 6, 9, and 12 mo), in the intervention arm and 10%, 11.5%, 18.4%, and 10.4% in the control arm (Figure 3). Results from interviews and field notes across all three sites suggested that some participants were taking rescue medications every month, regardless of whether they had an exacerbation. We observed that some participants were taking the rescue packs preventatively or not completing the full course of medications.
“One [CHW] mentioned how one of the patients … always takes the medicine as soon as he is given them and takes them irrespective of whether he has an exacerbation or not.” (meeting notes, Uganda).
“[Participant] doesn’t understand rescue pack usage and purpose. Wants to take steroids preventatively to help him when he leaves home.” (field notes, Nepal)
Fidelity
Results from the fidelity checklists demonstrate that CHWs had good adherence to protocol standards during observation visits (online supplement). Field notes and interviews showed that CHWs sometimes had challenges or forgot to emphasize the differences between the two yellow zones on the action plan. Furthermore, although some CHWs were comfortable correcting medication misuse, others did not have the confidence to correct those behaviors. Overall, the CHWs exhibited excellent interpersonal skills and work ethics and excelled most at providing emotional and social support during the visits.
Discussion
We conducted a pilot effectiveness-implementation randomized field trial of a multicomponent, CHW-supported self-management COPD action plan in a group of 239 participants with grades B–D COPD living in three low-resource settings in Nepal, Peru, and Uganda to improve respiratory health quality of life and reduce hospitalizations and exacerbations. We found no evidence of differences in respiratory health quality of life between the intervention and control arms, suggesting that proceeding to a larger trial with the currently proposed strategy is not warranted. Moderate to severe exacerbations, as defined by use of treatment, were more commonly documented in the intervention arm than in the control arm. Although the results of our trial cannot differentiate between a failed intervention or a failed implementation of the intervention, they suggest that the strategy across our settings should be revised.
Self-management interventions for COPD that include action plans have shown variable results for improving respiratory disease–related quality of life and respiratory-related hospital admissions. However, pooled analyses have shown that such interventions can lead to improvements in these outcomes (3, 27). Aboumatar and colleagues examined the effect of a transitional care and long-term self-management support intervention after discharge for individuals hospitalized for COPD in Baltimore, Maryland (28). Like in our study, the investigators found a higher proportion of COPD-related hospitalizations and emergency room visits in the intervention arm without improvement in quality of life. One possible explanation for the higher number of hospitalizations in the intervention arm in both studies is a heightened awareness of symptoms, as well as increased self-initiated healthcare use. In our study, close communication with a CHW may have led to increased referrals to acute care services and appropriately higher use of rescue packs. The higher dropout rate among control participants than among intervention participants may also have led to attrition bias, whereby individuals in the control arm with more severe illness and higher hospitalization rate were lost to follow-up and therefore were not captured in our analyses. One potential explanation for the differential dropout is that individuals in the control condition may have lost interest and perceived less of a direct benefit, given that they received only the initial COPD education visit and access to medications by going to a local distribution point. A second potential explanation is that individuals in the control arm had poorer COPD control, and thus their ability to continue in the study was compromised.
Administrative records, interviews, and field notes suggested that rescue packs were used more often by intervention participants than by control participants. The availability of rescue packs in the home has the potential to facilitate access to timely treatment for a COPD exacerbation, particularly for individuals with functional limitations that might restrict their ability to leave their homes to procure medications. However, this availability increases the likelihood of medication overuse. It is challenging to differentiate appropriate use from overuse in community-based studies. Midway through the trial, in response to several reports of suspected rescue pack overuse, we instituted a protocol whereby individuals requesting a rescue pack for three consecutive months no longer received automatic refills for rescue packs, but rather had to request one from their CHW. The CHW would provide further rescue pack education and then refer the participant to a physician for evaluation to ensure that a refill was appropriate. Of note, the medications included in the rescue packs (antibiotics, steroids) are generally available and comparatively affordable in local pharmacies in all three settings (17). This occurrence highlights the importance of training CHWs to provide oversight of medication use and availability of a qualified clinician to provide additional expertise as needed. Considering the limited healthcare infrastructure for treating COPD in these settings and the challenges with providing clinical oversight, the role of the CHW and clinician in overseeing treatment should be carefully delineated. In addition, as demonstrated by the low baseline use of inhaled preventative medications in this population, there is a clear need for expanded, facilitated access to long-term COPD medications in low-resource settings in LMICs (17, 29) at the health system level.
Strengths and Limitations
Our study has several strengths. This is one of few studies to implement a COPD self-management intervention in LMICs. Evaluating the multicomponent self-management intervention in multiple settings allows exploration of clinical outcomes as well as implementation across diverse communities in LMICs. Given the context-specific challenges and the disproportionate burden of disease in LMICs, this is an important strength. However, our study also has important limitations. Our study was not powered to detect differences in clinical outcomes at each of the study sites; rather, the study was designed to inform the decision to proceed to a larger future trial. Furthermore, the higher percentage with group D COPD in the intervention arm could have led to a bias toward the null; future studies should stratify enrollment by disease severity. We did not collect data on the availability of primary care and pulmonary physicians. Although there was a suggestion that antibiotics and steroids may have been overused among some participants, we were not able to evaluate whether there was an increased risk of infection as a result. The intervention did not include inhalers, but rather focused on training and support to identify COPD exacerbations and make informed decisions on when to seek care. The addition of inhaler education would be beneficial for any future iterations of this program, although access to affordable medications at these sites is limited. Finally, there was a larger proportion of participants who died or were lost to follow-up in the control arm when compared with the intervention arm. Despite following standard-of-care practices for control participants, it is possible that disparities in services offered to participants between the intervention and control arms may have contributed to differential dropout. Future studies should provide incentives to mitigate this problem.
There are aspects of our intervention design and strategies for implementation that merit discussion. In-depth interviews with participants and CHWs suggested that the regular visits and follow-up provided by CHWs were, for many participants, a welcome source of support and education. Many CHWs found satisfaction in providing this support, whereas others saw it as an additional burden on their existing responsibilities. Self-reported adoption of the COPD action plan across settings was moderate (generally less than 50%), highest in Peru, and lowest in Nepal. Given that we measured use of the action plan via self-report, it is likely that actual use was lower. Interviews, observations, and administrative records as part of the process evaluation (forthcoming) suggest that the design and mode of delivery for the action plan (e.g., didactic vs. interactive), as well as the user interface of our rescue packaging, could have benefited from a more rigorous, iterative design process that employs methods and principles from user-centered design.
Inclusion of task-shifting strategies to support individuals in self-management and linkage to care has the potential to overcome many structural limitations in the health system. However, the effectiveness of self-management interventions such as the one tested in this study, whether delivered via task shifting or otherwise, will be limited by the health system, economic, and geopolitical contexts in which they are implemented. For example, both Nepal and Uganda have larger CHW networks that perform home visits as part of their regular duties and are compensated. In Peru, CHW networks are smaller and often hired for shorter-term programs, such as vaccination campaigns or care of the elderly. These and other factors, such as existing workload and specific role within the overall health system, would influence intervention fit or required adaptations in a particular setting. Furthermore, in the absence of COPD medication availability (3, 17), CHWs would be unable to carry out their duties in the distribution of evidence-based treatments. As such, task-shifting strategies should complement larger-scale structural reforms to improve the quality and accessibility of chronic disease treatment and care.
Conclusions
We found that a multicomponent COPD self-management intervention supported by CHWs did not improve disease-specific quality of life and resulted in a higher proportion of treated moderate to severe exacerbations defined by the use of rescue packs, with the suggestion of overuse, compared with standard of care plus COPD education. We performed this intervention across three low-resource community settings in three different continents that varied in culture, level of urbanization, and healthcare infrastructure and resources. Interventions and accompanying implementation strategies should be carefully adapted to the social, structural, and systemic contexts in which they are performed. This pilot trial, although not definitive, provides insight into the important considerations and challenges for implementing COPD self-management interventions in such contexts, where diagnosis and treatment gaps are vast and availability of and access to evidence-based treatments are limited. Special attention should be paid to the potential for antibiotic and oral steroid overuse in self-management interventions for COPD. CHW or other task-shifting models have the potential to improve detection, linkage, and treatment outcomes for people living with COPD in LMICs. However, their effectiveness will be limited without complementary structural and system-level interventions to address inequities in the quality, availability, and affordability of COPD care across the globe.
Acknowledgments
Acknowledgment
The authors are grateful for the contributions of the field research teams, participants in the trial, the communities in which the trial took place, the trial steering committee, the Medical Research Council, and the Global Alliance for Chronic Disease research network. The authors also acknowledge Dr. Lindsay J. Underhill for providing insightful comments on the manuscript.
Footnotes
Other GECo2 Trial Investigators: Susan Michie, D.Phil., Phil.C.Psychol., University College London, London, United Kingdom; Zachos Anastasiou, M.Sc., University College London, London, United Kingdom; Nicole Robertson, M.D., Johns Hopkins University, Baltimore, Maryland; Robert A Wise, M.D., Johns Hopkins University, Baltimore, Maryland; Karbir N. Yogi, M.D., Institute of Medicine, Kathmandu Nepal; Denis Mawanda, B.S., Makerere University, Kampala, Uganda; Faith Nassali, B.S., Makerere University, Kampala, Uganda; Robert Kalyesubula M.B. Ch.B., M.Med., Ph.D., Makerere University, Kampala, Uganda; Elisa Romani-Huacani, R.N., M.Sc., Asociación Benéfica PRISMA, Lima, Peru; and Adithya Cattamanchi, M.D., M.A.S., University of California, Irvine, Orange, California.
Supported by the Medical Research Council (grant MR/POO8984/1) and by the National Institute for Health Research using U.K. aid from the U.K. government to support global health research. S.L.P. was supported by a Mentored Research Scientist Development Award and T.S. was supported by a Mentored Patient-oriented Research Career Development Award, both from the NHLBI, NIH (1K01HL140048 and K23HL126946). OFF and WC were also supported in part by a Research training grant in chronic, non-communicable respiratory diseases in Peru (D43TW01152). The views expressed in this publication are those of the author(s) and not necessarily those of the Medical Research Council, the National Institute for Health Research, the National Institutes of Health, or the U.K. or U.S. government.
Author Contributions: Concept and design: S.L.P., T.S., J.A.B., B.K., J.J.M., W.C., and J.R.H. Acquisition, analysis, or interpretation of data: S.L.P., T.S., S.H., N.A.R., O.F.-F., P.A., S.Q., I.A., J.A.B., R.C., S.K.D., G.G., B.K., K.G., S.M., F.R., A.K.S., L.S., M.O.S., A.C.W., J.R.H., and W.C. Drafting of the manuscript: S.L.P., T.S., S.H., W.C., and J.R.H. Critical revision of the manuscript for important intellectual content: S.L.P., T.S., S.H., N.A.R., O.F.-F., P.A., S.Q., I.A., J.A.B., R.C., S.K.D., G.G., B.K., K.G., J.J.M., S.M., F.R., A.K.S., L.S., M.O.S., A.C.W., J.R.H., and W.C. Obtained funding: S.L.P., T.S., J.A.B., B.K., J.J.M., W.C., and J.R.H. Administrative, technical, or material support: S.L.P., T.S., N.A.R., O.F.-F., P.A., S.Q., I.A., J.A.B., R.C., S.K.D., G.G., B.K., K.G., J.J.M., S.M., F.R., A.K.S., L.S., M.O.S., A.C.W., J.R.H., and W.C. Supervision: T.S., S.L.P., S.Q., N.A.R., A.C.W., P.A., J.A.B., R.C., O.F.-F., B.K., J.J.M., A.K.S., S.K.D., L.S., M.O.S., W.C., and J.R.H.
Data sharing: Individual participant data that underlie the results reported in this article, after deidentification, will be shared upon request by the study authors with researchers who provide a methodologically sound proposal. The data will be available beginning 9 months and ending 36 months after article publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202303-0505OC on September 12, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
for the GECo2 Trial Investigators:
Susan Michie, Zachos Anastasiou, Nicole Robertson, Robert A Wise, Karbir N. Yogi, Denis Mawanda, Faith Nassali, Robert Kalyesubula, Elisa Romani-Huacani, and Adithya Cattamanchi
References
- 1.World Health Organization. 2023. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- 2. Hurst JR, Buist AS, Gaga M, Gianella GE, Kirenga B, Khoo EM, et al. Challenges in the implementation of chronic obstructive pulmonary disease guidelines in low- and middle-income countries: an official American Thoracic Society workshop report. Ann Am Thorac Soc . 2021;18:1269–1277. doi: 10.1513/AnnalsATS.202103-284ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Florman KE, Siddharthan T, Pollard SL, Alupo P, Barber JA, Chandyo RK, et al. GECo Study Investigators Unmet diagnostic and therapeutic opportunities for chronic obstructive pulmonary disease in low- and middle-income countries. Am J Respir Crit Care Med . 2023;208:442–450. doi: 10.1164/rccm.202302-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lenferink A, Brusse-Keizer M, van der Valk PD, Frith PA, Zwerink M, Monninkhof EM, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2017;8:CD011682. doi: 10.1002/14651858.CD011682.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CE, Wood JJ. In: Encyclopedia of autism spectrum disorders. Volkmar FR, editor. New York: Springer; 2013. Self-management interventions; pp. 2735–2743. [Google Scholar]
- 6. Kaplan A. The COPD action plan. Can Fam Physician . 2009;55:58–59. [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi R, Alim M, Kengne AP, Jan S, Maulik PK, Peiris D, et al. Task shifting for non-communicable disease management in low and middle income countries—a systematic review. PLoS One . 2014;9:e103754. doi: 10.1371/journal.pone.0103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maria JL, Anand TN, Dona B, Prinu J, Prabhakaran D, Jeemon P. Task-sharing interventions for improving control of diabetes in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Health . 2021;9:e170–e180. doi: 10.1016/S2214-109X(20)30449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He J, Irazola V, Mills KT, Poggio R, Beratarrechea A, Dolan J, et al. HCPIA Investigators Effect of a community health worker-led multicomponent intervention on blood pressure control in low-income patients in Argentina: a randomized clinical trial. JAMA . 2017;318:1016–1025. doi: 10.1001/jama.2017.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parekh TM, Copeland CR, Dransfield MT, Cherrington A. Application of the community health worker model in adult asthma and COPD in the U.S.: a systematic review. BMC Pulm Med . 2019;19:116. doi: 10.1186/s12890-019-0878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis . 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 12. Siddharthan T, Pollard SL, Quaderi SA, Mirelman AJ, Cárdenas MK, Kirenga B, et al. GECo Study Investigators Effectiveness-implementation of COPD case finding and self-management action plans in low- and middle-income countries: global excellence in COPD outcomes (GECo) study protocol. Trials . 2018;19:571. doi: 10.1186/s13063-018-2909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14. Siddharthan T, Pollard SL, Quaderi SA, Rykiel NA, Wosu AC, Alupo P, et al. GECo Study Investigators Discriminative accuracy of chronic obstructive pulmonary disease screening instruments in 3 low- and middle-income country settings. JAMA . 2022;327:151–160. doi: 10.1001/jama.2021.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagourney EM, Robertson NM, Rykiel N, Siddharthan T, Alupo P, Encarnación M, et al. GECo Study Investigators Illness representations of chronic obstructive pulmonary disease (COPD) to inform health education strategies and research design-learning from rural Uganda. Health Educ Res . 2020;35:258–269. doi: 10.1093/her/cyaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. https://goldcopd.org/wp-content/uploads/2017/02/wms-GOLD-2017-FINAL.pdf
- 17. Siddharthan T, Robertson NM, Rykiel NA, Underhill LJ, Rahman N, Kafle S, et al. Availability, affordability and access to essential medications for asthma and chronic obstructive pulmonary disease in three low- and middle- income country settings. PLoS Glob Public Health . 2022;2:e0001309. doi: 10.1371/journal.pgph.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sealed Envelope. https://www.sealedenvelope.com
- 19. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci . 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrer M, Alonso J, Prieto L, Plaza V, Monsó E, Marrades R, et al. Validity and reliability of the St George’s Respiratory Questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J . 1996;9:1160–1166. doi: 10.1183/09031936.96.09061160. [DOI] [PubMed] [Google Scholar]
- 21.Morgan BW, Grigsby MR, Siddharthan T, Kalyesubula R, Wise RA, Hurst JR, et al. Validation of the Saint George’s Respiratory Questionnaire in Uganda. BMJ Open Respir Res. 2018;5:e000276. doi: 10.1136/bmjresp-2018-000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherpa CT, LeClerq SL, Singh S, Naithani N, Pangeni R, Karki A, et al. Validation of the St. George’s Respiratory Questionnaire in Nepal. Chronic Obstr Pulm Dis (Miami) . 2015;2:281–289. doi: 10.15326/jcopdf.2.4.2014.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooks R. EuroQol: the current state of play. Health Policy . 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 24. Cocks K, Torgerson DJ. Sample size calculations for pilot randomized trials: a confidence interval approach. J Clin Epidemiol . 2013;66:197–201. doi: 10.1016/j.jclinepi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 25. Welling JB, Hartman JE, Ten Hacken NH, Klooster K, Slebos DJ. The minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPD. Eur Respir J . 2015;46:1598–1604. doi: 10.1183/13993003.00535-2015. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. https://www.R-project.org/ [Google Scholar]
- 27. Schrijver J, Lenferink A, Brusse-Keizer M, Zwerink M, van der Valk PD, van der Palen J, et al. Self-management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2022;1:CD002990. doi: 10.1002/14651858.CD002990.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA . 2019;322:1371–1380. doi: 10.1001/jama.2019.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robertson NM, Nagourney EM, Pollard SL, Siddharthan T, Kalyesubula R, Surkan PJ, et al. Urban-rural disparities in chronic obstructive pulmonary disease management and access in Uganda. Chronic Obstr Pulm Dis (Miami) . 2019;6:17–28. doi: 10.15326/jcopdf.6.1.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Bank. 2018. https://data.worldbank.org/