From the Authors:
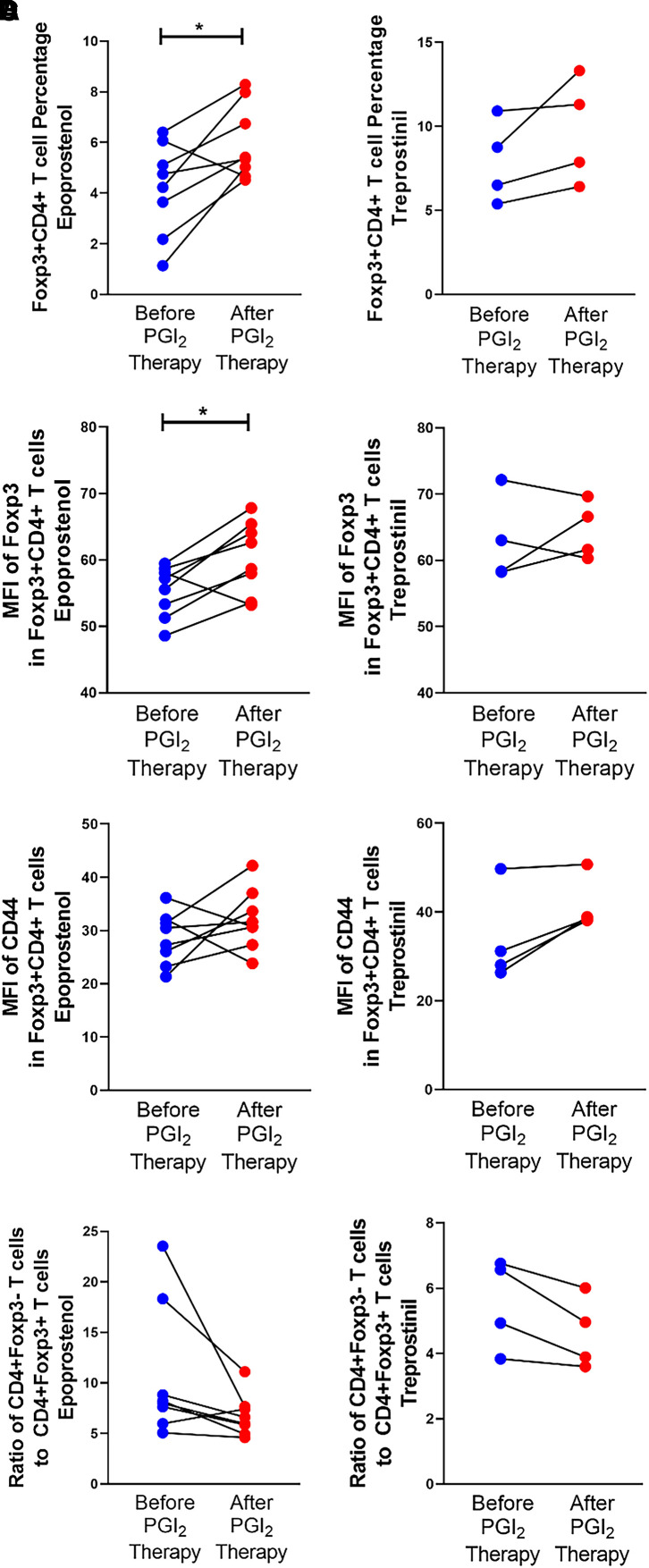
We thank Yasuma and colleagues for their interest in our study recently published in the Journal in which we demonstrated that prostaglandin I2 (PGI2) analogues increased the percentage of circulating T regulatory cells (Tregs), the mean fluorescence intensity (MFI) of Foxp3 within the Tregs, and the MFI of CD44 on the Tregs while decreasing the ratio of T effector cells to Tregs in patients with pulmonary arterial hypertension (1). The authors commented on the potential differential effects that different PGI2 analogues may have on Treg generation in humans. Eight patients were treated with epoprostenol and four were treated with treprostinil during the study. It is important to note that the patients in this study received a room-temperature stable formulation of epoprostenol or a room-temperature stable formulation of treprostinil (2, 3). In response to the concerns of Yasuma and colleagues that these different analogues may have differential effects, we have separated the data for each of our endpoints based on treatment. We found significant increases in Treg percentage (Figure 1A) and Foxp3 MFI (Figure 1B) in patients treated with epoprostenol, but not those treated with treprostinil, after grouping the patients based on treatment received. No differences in CD44 MFI (Figure 1C) or in the ratio of CD4+ T effector cells to Treg (Figure 1D) were observed after grouping patients based on treatment received. However, we are underpowered for this subgroup analysis, and there may be selection bias in treatment recommendations that cannot be accounted for by the low number of subjects in each group. Therefore, we cannot confidently conclude whether there are differential effects on Treg generation and function by the different PGI2 analogues. We appreciate this question and will consider this while planning a larger follow-up study.
Figure 1.
Effect of the prostaglandin I2 analogues epoprostenol or treprostinil on (A) T regulatory cell (Treg) (Foxp3+CD4+) percentage, (B) Foxp3 mean fluorescence intensity in Tregs, (C) CD44 mean fluorescence intensity in Tregs, and (D) ratio of T effector cells (CD4+Foxp3−) to Tregs (CD4+Foxp3+). Analysis was performed with a paired t test. *P < 0.05. MFI = mean fluorescence intensity; PGI2 = prostaglandin I2.
Footnotes
Supported by NIH grants K99 HL159594 (A.E.N.), R01 AI124456 (R.S.P.), R01 AI145265 (R.S.P.), U19 AI095227 (R.S.P.), R01 AI111820 (R.S.P.), R21 AI145397 (R.S.P.), and R01 HL142720 (A.R.H.); Division of Intramural Research, National Institute of Allergy and Infectious Diseases grant F32 AI143005 (A.E.N.); NHLBI grant K24 HL155891 (A.R.H.); Biomedical Laboratory Research and Development, VA Office of Research and Development grant 101BX004299 (R.S.P.); and Vanderbilt Institute for Clinical and Translational Research grants VR55954 and VR66842 (A.E.N.).
Originally Published in Press as DOI: 10.1164/rccm.202309-1622LE on September 29, 2023
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Norlander AE, Abney M, Cephus JY, Roe CE, Irish JM, Shelburne NJ, et al. Prostaglandin I2 therapy promotes regulatory T cell generation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med . 2023;208:737–739. doi: 10.1164/rccm.202304-0716LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greig SL, Scott LJ, Plosker GL. Epoprostenol (Veletri®, Caripul®): a review of its use in patients with pulmonary arterial hypertension. Am J Cardiovasc Drugs . 2014;14:463–470. doi: 10.1007/s40256-014-0093-0. [DOI] [PubMed] [Google Scholar]
- 3. Kumar P, Thudium E, Laliberte K, Zaccardelli D, Nelsen A. A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet . 2016;55:1495–1505. doi: 10.1007/s40262-016-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]