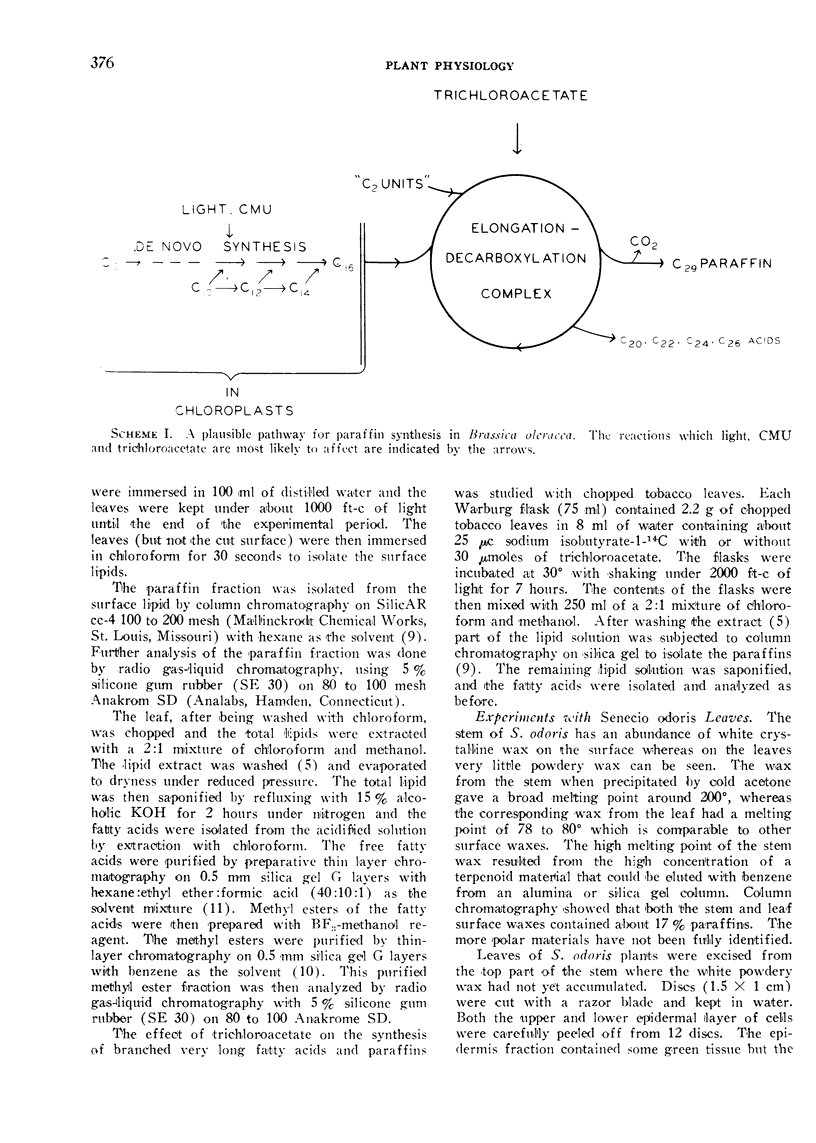
Abstract
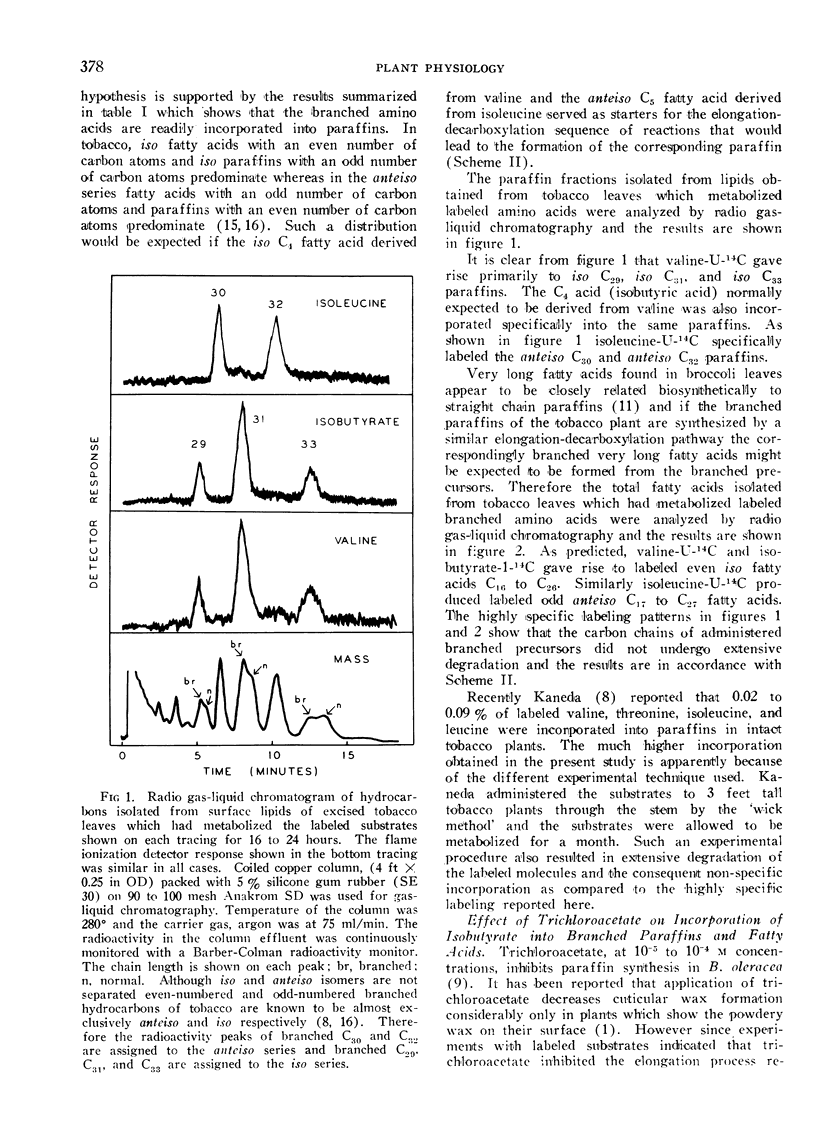
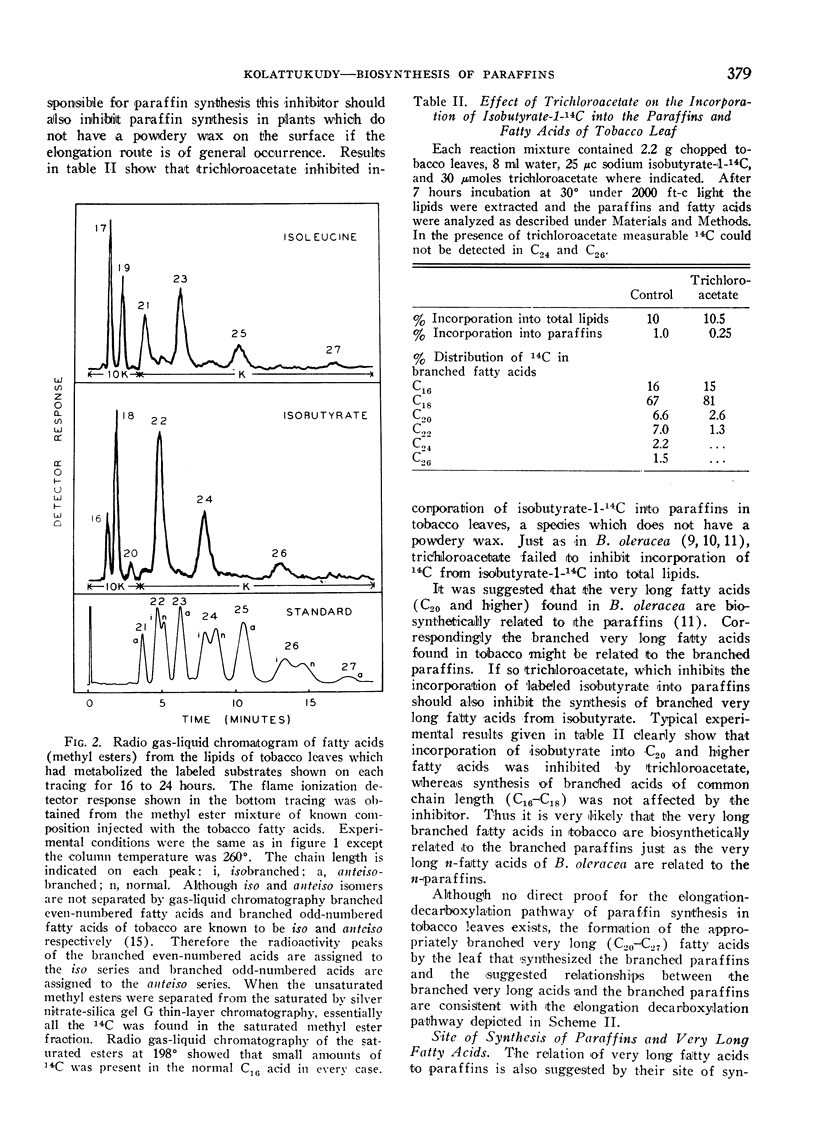
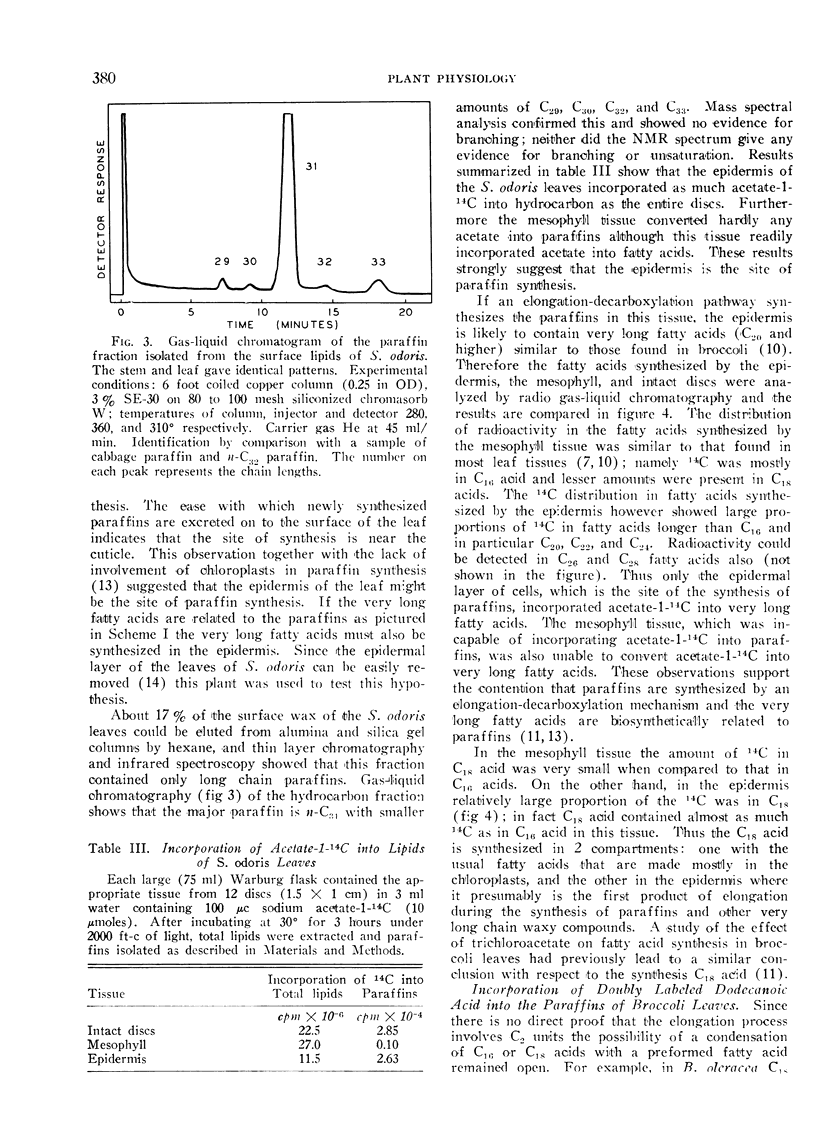
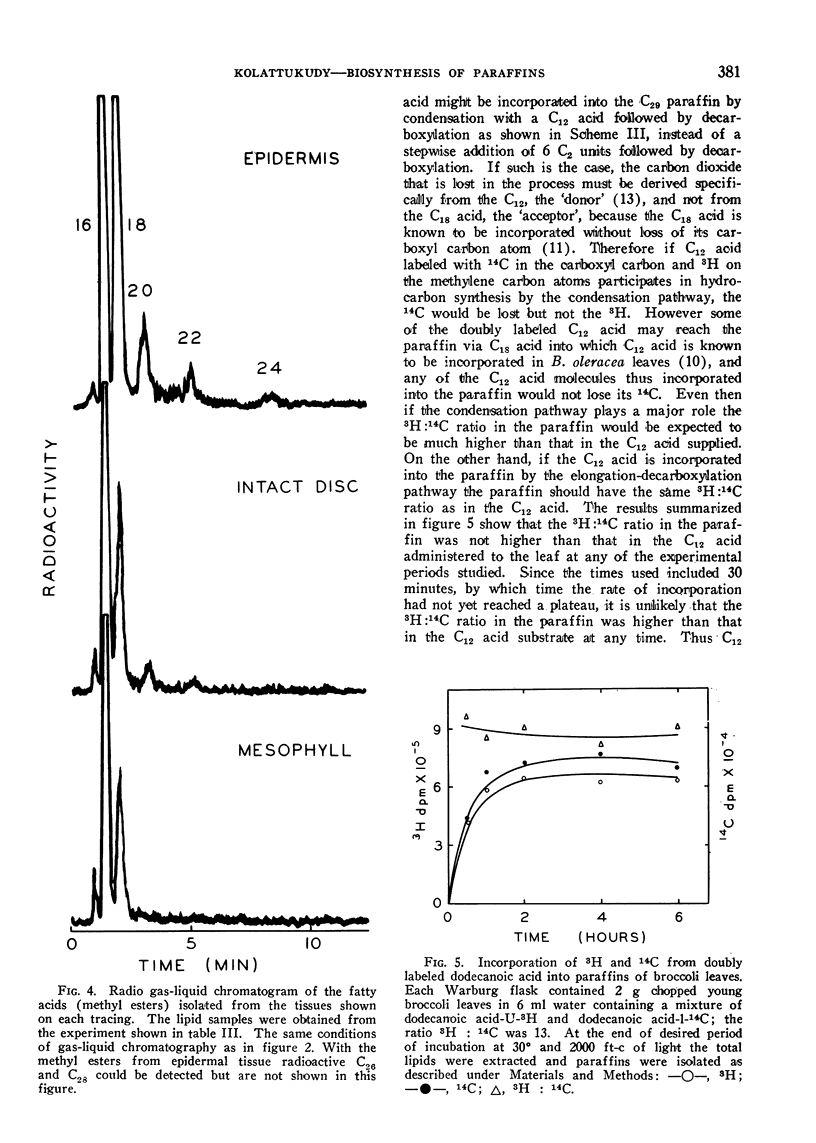
In isolated tobacco leaves l-valine-U-14C gave rise to labeled even-numbered isobranched fatty acids containing 16 to 26 carbon atoms and iso C29, iso C31, and iso C33 paraffins. l-Isoleucine-U-14C on the other hand produced labeled odd-numbered anteiso C17 to C27 fatty acids and anteiso C30 and C32 paraffins. Trichloroacetic acid inhibited the incorporation of isobutyrate into C20 and higher fatty acids and paraffins without affecting the synthesis of the C16 and C18 fatty acids. Thus the very long branched fatty acids are biosynthetically related to the paraffins. In Senecio odoris leaves acetate-1-14C was incorporated into the paraffins (mainly n-C31) only in the epidermis although acetate was readily incorporated into fatty acids in the mesophyll tissue. Similarly only the epidermal tissue incorporated acetate into fatty acids longer than C18 suggesting that the epidermis is the site of synthesis of both paraffins and the very long fatty acids. In broccoli leaves n-C12 acid labeled with 14C in the carboxyl carbon and 3H in the methylene carbons was incorporated into C29 paraffin without the loss of 14C relative to 3H. Since n-C18 acid is known to be incorporated into the paraffin without loss of carboxyl carbon these results suggest that the condensation of C12 acid with C18 acid is not responsible for n-C29 paraffin synthesis in this tissue. Thus all the experimental evidence thus far obtained strongly suggests that elongation of fatty acids followed by decarboxylation is the most likely pathway for paraffin biosynthesis in leaves.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eglinton G., Hamilton R. J. Leaf epicuticular waxes. Science. 1967 Jun 9;156(3780):1322–1335. doi: 10.1126/science.156.3780.1322. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HORNING M. G., MARTIN D. B., KARMEN A., VAGELOS P. R. Fatty acid synthesis in adipose tissue. II. Enzymatic synthesis of branched chain and odd-numbered fatty acids. J Biol Chem. 1961 Mar;236:669–672. [PubMed] [Google Scholar]
- JAMES A. T. The biosynthesis of long-chain saturated and unsaturated fatty acids in isolated plant leaves. Biochim Biophys Acta. 1963 Feb 19;70:9–19. doi: 10.1016/0006-3002(63)90714-5. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of long-chain hydrocarbons. I. Incorporation of L-valine, L-threonine, L-isoleucine, and L-leucine into specific branched-chain hydrocarbons in tobacco. Biochemistry. 1967 Jul;6(7):2023–2032. doi: 10.1021/bi00859a021. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthesis of surface lipids. Biosynthesis of long-chain hydrocarbons and waxy esters is discussed. Science. 1968 Feb 2;159(3814):498–505. doi: 10.1126/science.159.3814.498. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthesis of wax in Brassica oleracea. Relation of fatty acids to wax. Biochemistry. 1966 Jul;5(7):2265–2275. doi: 10.1021/bi00871a015. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Mechanisms of synthesis of waxy esters in broccoli (Brassica oleracea). Biochemistry. 1967 Sep;6(9):2705–2717. doi: 10.1021/bi00861a010. [DOI] [PubMed] [Google Scholar]
- Kuiper P. J. Dependence upon Wavelength of Stomatal Movement in Epidermal Tissue of Senecio odoris. Plant Physiol. 1964 Nov;39(6):952–955. doi: 10.1104/pp.39.6.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLD J. D., STEVENS R. K., MEANS R. E., RUTH J. M. THE PARAFFIN HYDROCARBONS OF TOBACCO; NORMAL, ISO-, AND ANTEISO-HOMOLOGS. Biochemistry. 1963 May-Jun;2:605–610. doi: 10.1021/bi00903a037. [DOI] [PubMed] [Google Scholar]
- WHEATLEY V. R., CHOW D. C., KEENAN F. D., Jr Studies of the lipids of dog skin. II. Observations of the lipid metabolism of perfused surviving dog skin. J Invest Dermatol. 1961 Apr;36:237–239. doi: 10.1038/jid.1961.40. [DOI] [PubMed] [Google Scholar]


