Figure 3: Na-Cl polarization energy.
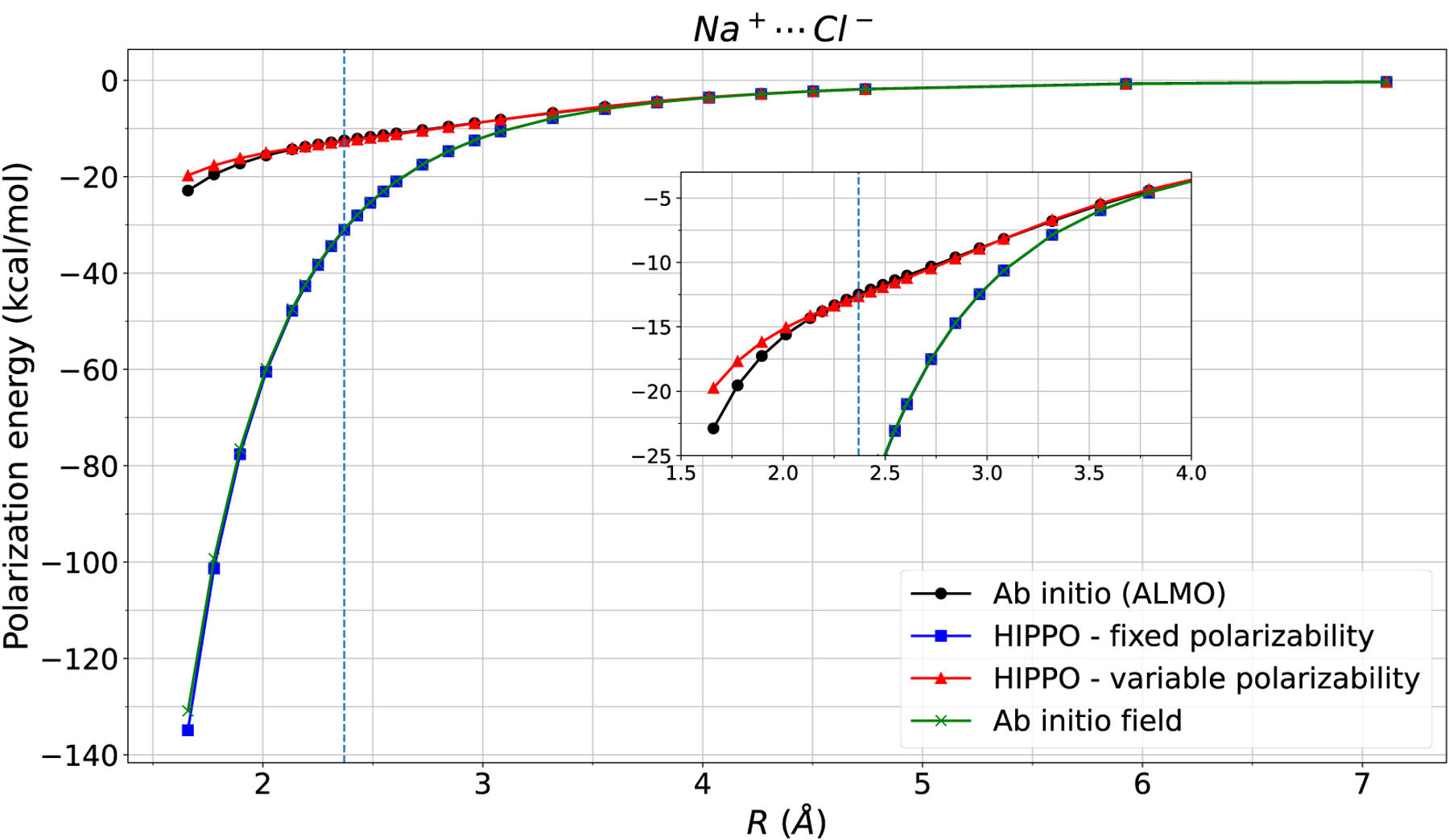
Polarization energy of the sodium chloride dimer is computed four different ways. The HIPPO variable polarizability model closely mirrors ALMO polarization energy, whereas the HIPPO fixed polarizability model matches the ab initio field polarization energy. The issue with over polarization in classical force fields lies in the polarizability, not the permanent electric field. The vertical line indicates equilibrium distance.
