Figure 7.
ATM-factor Spp1 contributes to lesion repair
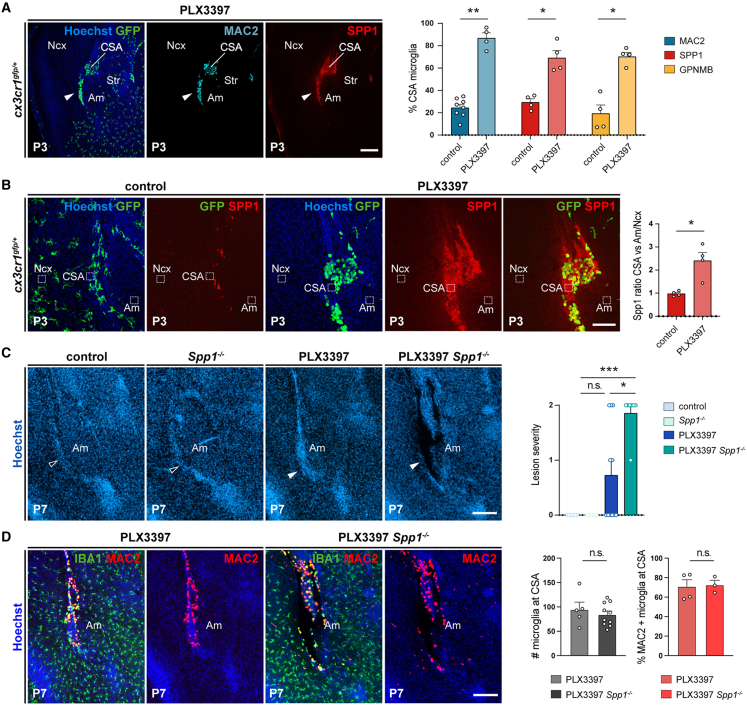
(A) Cx3cr1gfp-positive cells accumulating at the site of lesion closure co-express ATM markers Spp1, Mac2, and GPNMB, as shown and quantified at P3 (ncontrols = 4; nPLX3397 = 4 for each marker, from at least two distinct litters).
(B) Extracellular Spp1 signal, delineated by immunostaining and Hoechst labeling, accumulates at the resorbing CSA at P3. Graphs show the increased signal intensity at the CSA (dotted lines) compared with a mean between signal intensity measured in the surrounding neocortex (dotted lines) and amygdala (dotted lines) in PLX3397-exposed pups versus controls (ncontrols = 4; nPLX3397 = 4, from two distinct litters).
(C) In contrast to controls, Spp1−/− mutants, and PLX3397-exposed controls, PLX3397-exposed Spp1−/− mutants reproducibly displayed lesions visible by Hoechst staining (solid versus open arrowheads) (ncontrols = 7; nSpp1KO = 8; ncontrol-PLX3397 = 11; nSpp1KO-PLX3397 = 7). Values represent the scoring of lesion severity, scored from 0 to 2, as detailed in Table S2.
(D) While CSA IBA1-positive cells co-expressed Mac2 in resorbed PLX3397-treated controls at P7, they also accumulated around the lesions in PLX3397-treated Spp1 mutants, indicating that Spp1 inactivation did not prevent the expression of selected ATM markers (ncontrol-PLX3397 = 3; nSpp1KO-PLX3397 = 7).
Graphs show means ± SEM. Mann-Whitney U test was performed for statistical comparison, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, non significant (p > 0.5). Scale bars: 200 μm in (A); 150 μm in (C) and (D); and 100 μm in (B).
Am, amygdala; CSA, cortico-striato-amygdalar boundary; Ncx, neocortex; Str, striatum.
See also Figure S7.