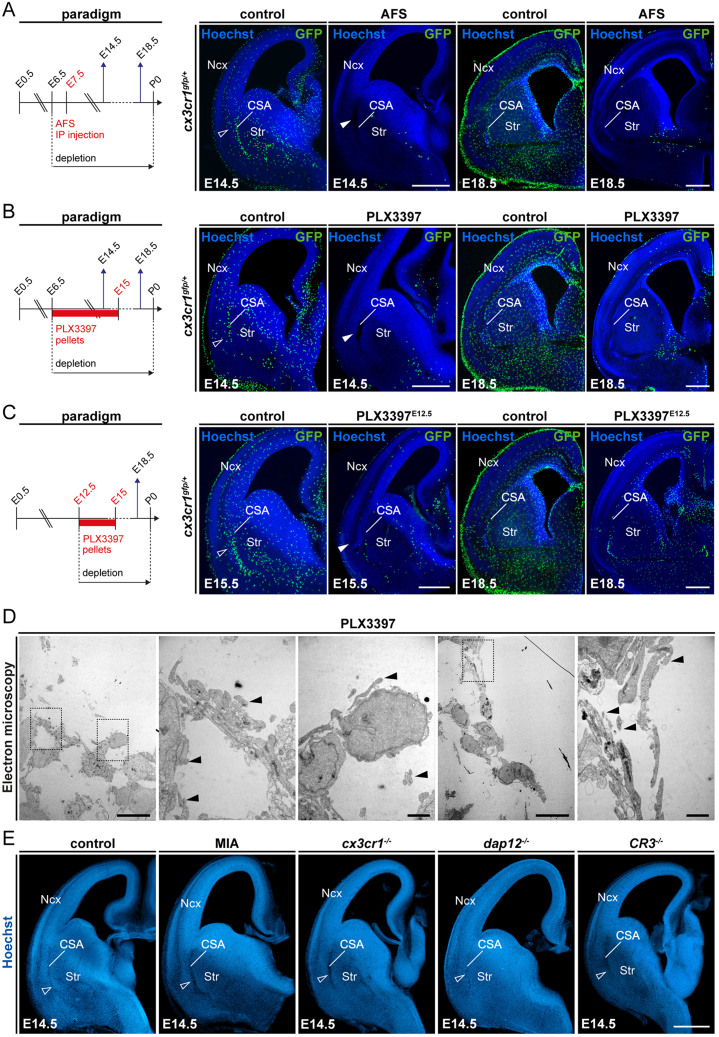
Figure S2.
Models of macrophage depletion and functional alteration, related to Figure 2
(A–C) Confirmation of the depletion of microglia in brains of embryonic Cx3cr1gfp/+ mice at E14.5 and E18.5, where pregnant dams were subjected to (A) intraperitoneal injection of a CSF1R blocking antibody (AFS) at E6.5 and E7.5 (E14.5, ncontrols = 21, nAFS = 6; E18.5, ncontrols = 15, nAFS = 17); (B) feeding with PLX3397, a pharmacological inhibitor of the CSF1R pathway, from E6.5 to E15.0 (E14.5, ncontrols = 5, nPLX3397 = 5; E18.5, ncontrols = 12, nPLX3397 = 11); and (C) feeding with PLX3397 from E12.5 to E15.0 (E15.5, ncontrols = 5, nPLX3397-E12 = 9; E18.5, ncontrols = 12, nPLX3397-E12 = 10). Open and solid arrowheads indicate the accumulation of GFP-positive cells at the CSA and local CSA tissue lesion in the absence of GFP-positive cells, respectively.
(D) Transmission electron microscopy image of the CSA lesion in the brain of E14.5 embryos from PLX3397-treated dams, showing the presence of cell debris (solid arrowheads) but the absence of basal membrane (n = 3).
(E) Coronal sections through hemibrains of E14.5 embryos from wild-type mice; those exposed to mild maternal immune activation (MIA); and Cx3cr1−/−, Dap12/TyroBP−/−, and CR3−/− mutant embryos showing the absence of CSA lesions (open arrowheads) (at least n = 6 for each condition).
Scale bars: 500 μm in (A)–(C), and (E); 10 μm in (D, low magnification); and 2 μm in (D, high magnification).
CSA, cortico-striato-amygdalar boundary; Ncx, neocortex; Str, striatum.