Abstract
Thermal reversion of the far-red absorbing form of phytochrome to the red absorbing form in darkness has been investigated in crude and partially purified isolates from a number of etiolated and light grown higher plants. The influence of temperature, aging and urea on the rate of reversion was also determined.
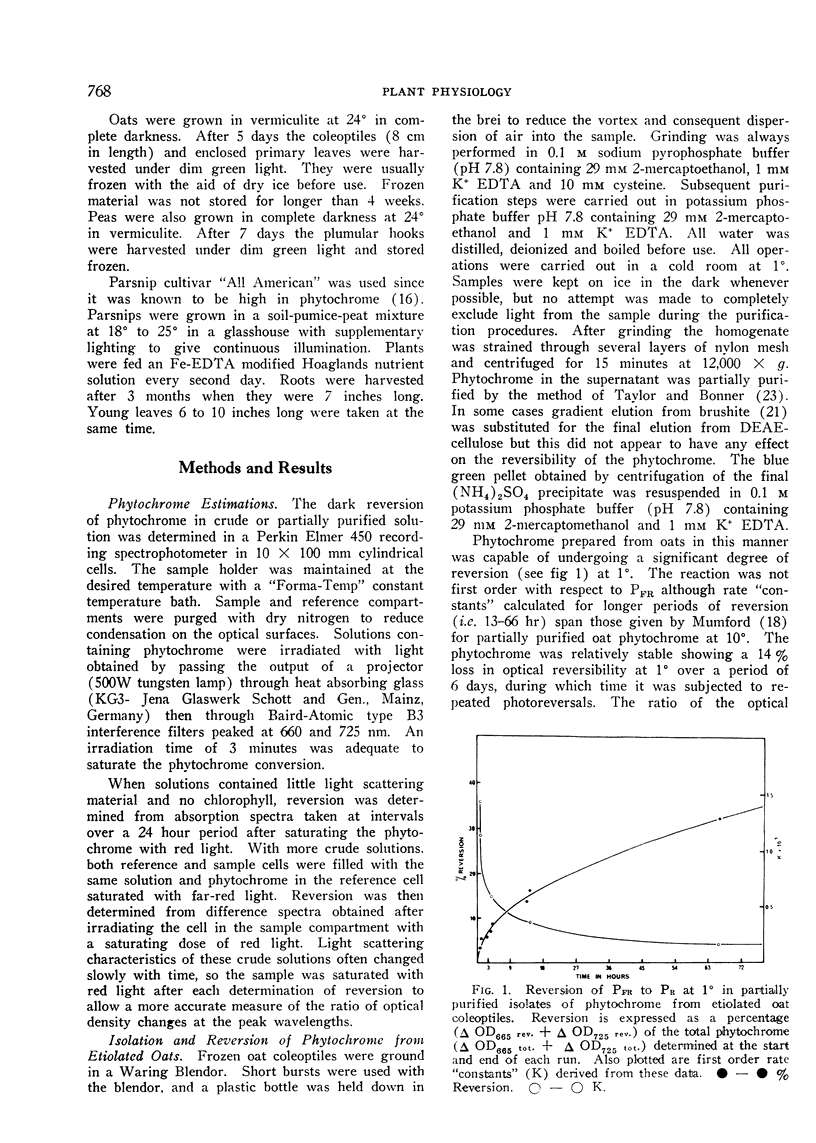
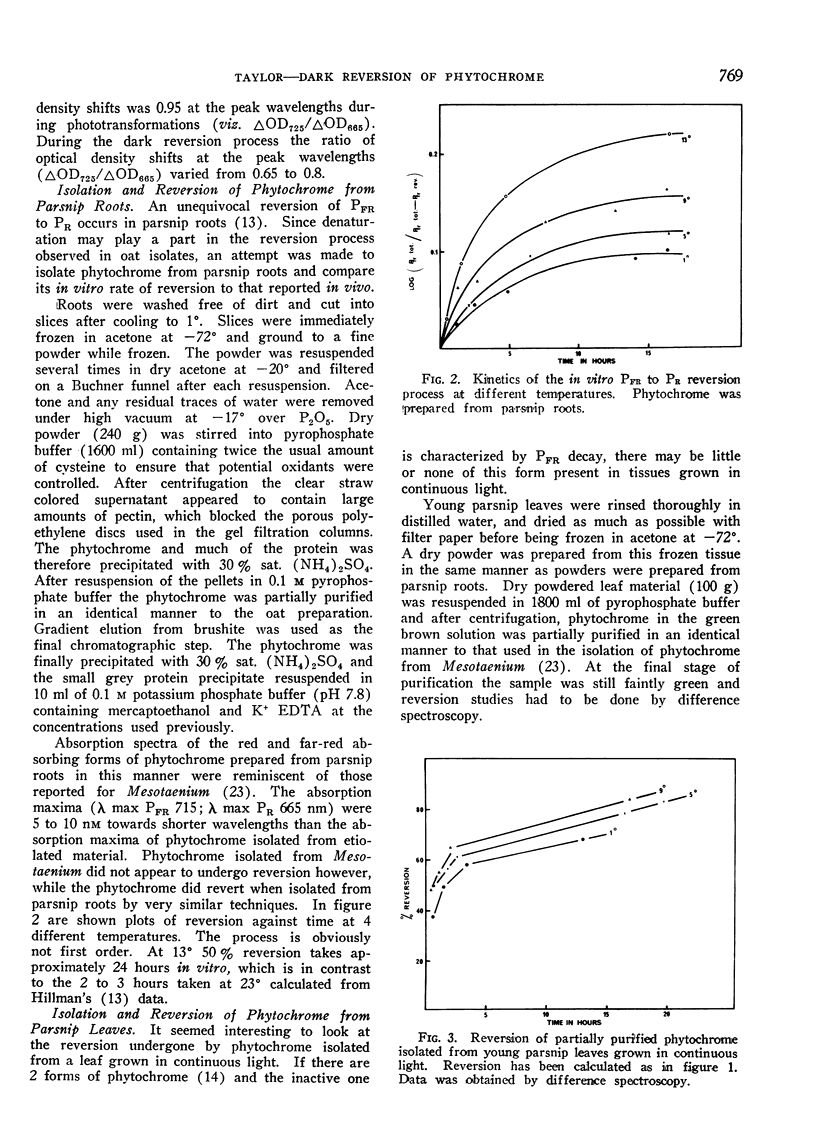
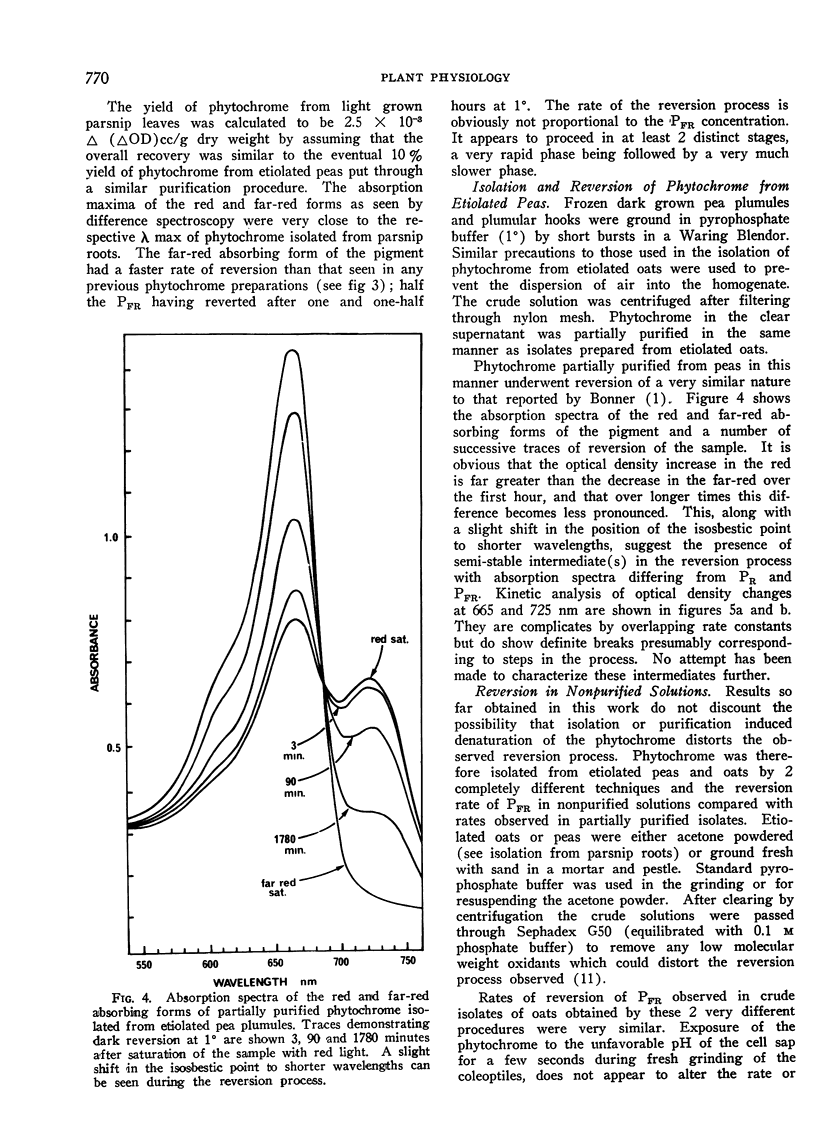
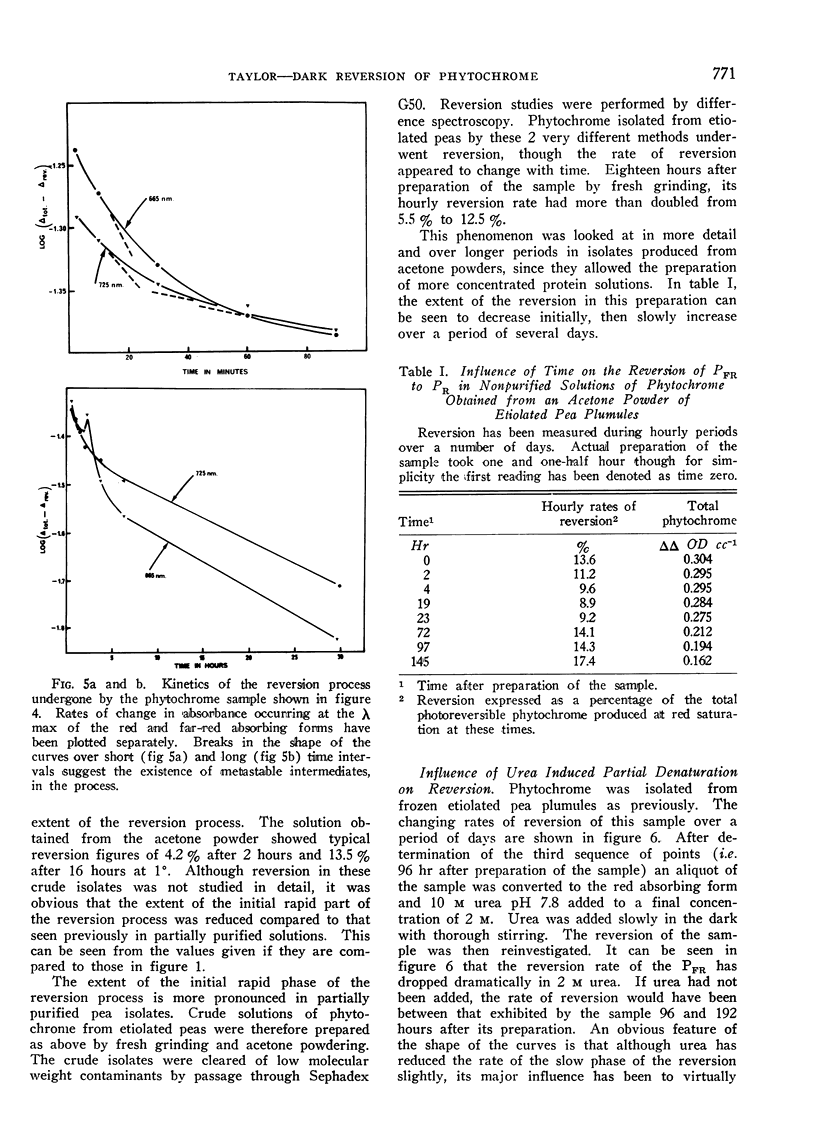
Phytochrome isolated from all higher plants underwent reversion. The reversion proceeded in at least 2 distinct stages; a short rapid initial phase being followed a slow phase which continued for many hours. Reversion rate was highest in phytochrome isolated from green leaves of parsnip (Pastinacea sativa) and lowest in that isolated from etiolated oats (Avena sativa). Although the rate of reversion could be changed by modifying the tertiary structure of the protein component, the large differences in rate appeared to be characteristic of the plant source. Observed in vitro rates of reversion are slower than those occurring in vivo. Removal of other buffer solubilized material during purification had little effect on the rate of reversion of phytochrome isolated from etiolated material.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borthwick H. A., Hendricks S. B., Parker M. W., Toole E. H., Toole V. K. A Reversible Photoreaction Controlling Seed Germination. Proc Natl Acad Sci U S A. 1952 Aug;38(8):662–666. doi: 10.1073/pnas.38.8.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Purification and properties of phytochrome: a chromoprotein regulating plant growth. Fed Proc. 1965 Jul-Aug;24(4):863–867. [PubMed] [Google Scholar]


