Abstract
The activity of invertase and its relation to growth were studied in the epicotyls of lentil seedlings incubated in the presence and absence of gibberellic acid (GA3).
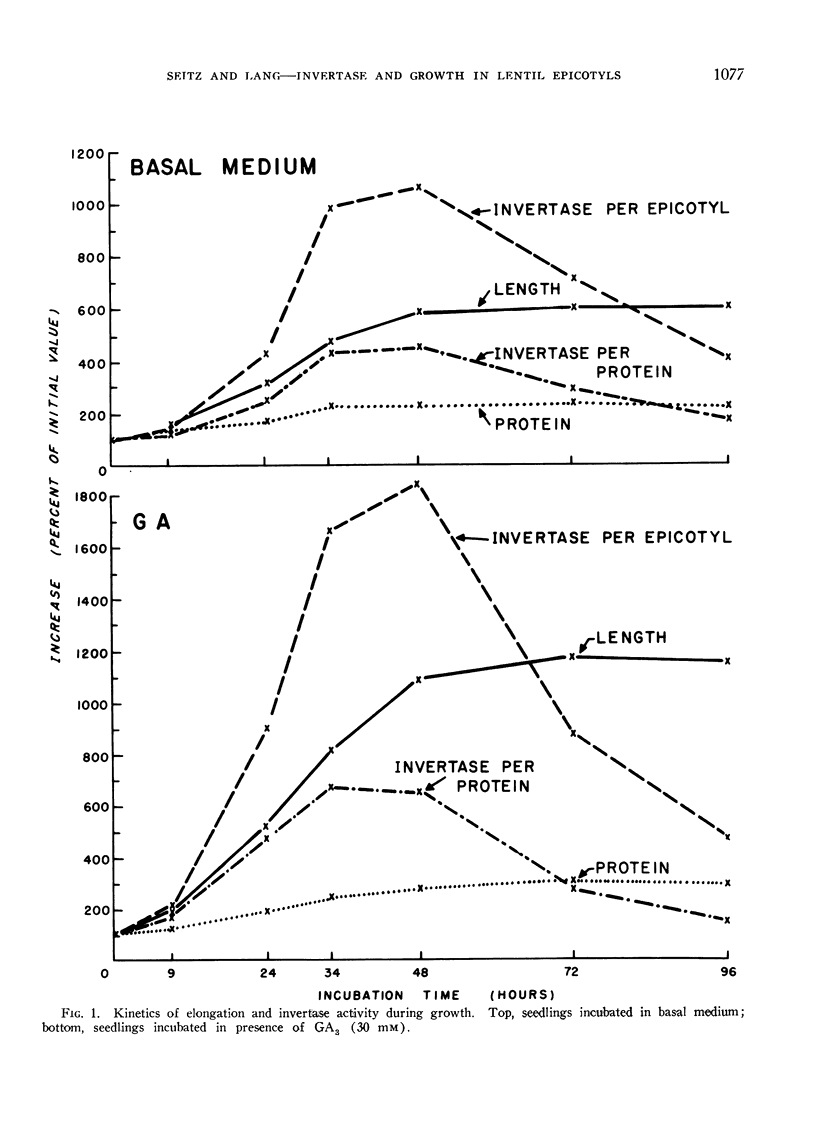
Invertase activity per epicotyl increases relatively more rapidly than does length, reaches a maximum during most active elongation, and declines upon cessation of growth.
GA3 enhances both growth and increase in invertase activity, without altering the kinetics of the 2 processes. If GA3 is added during incubation invertase activity increases more rapidly than does elongation rate.
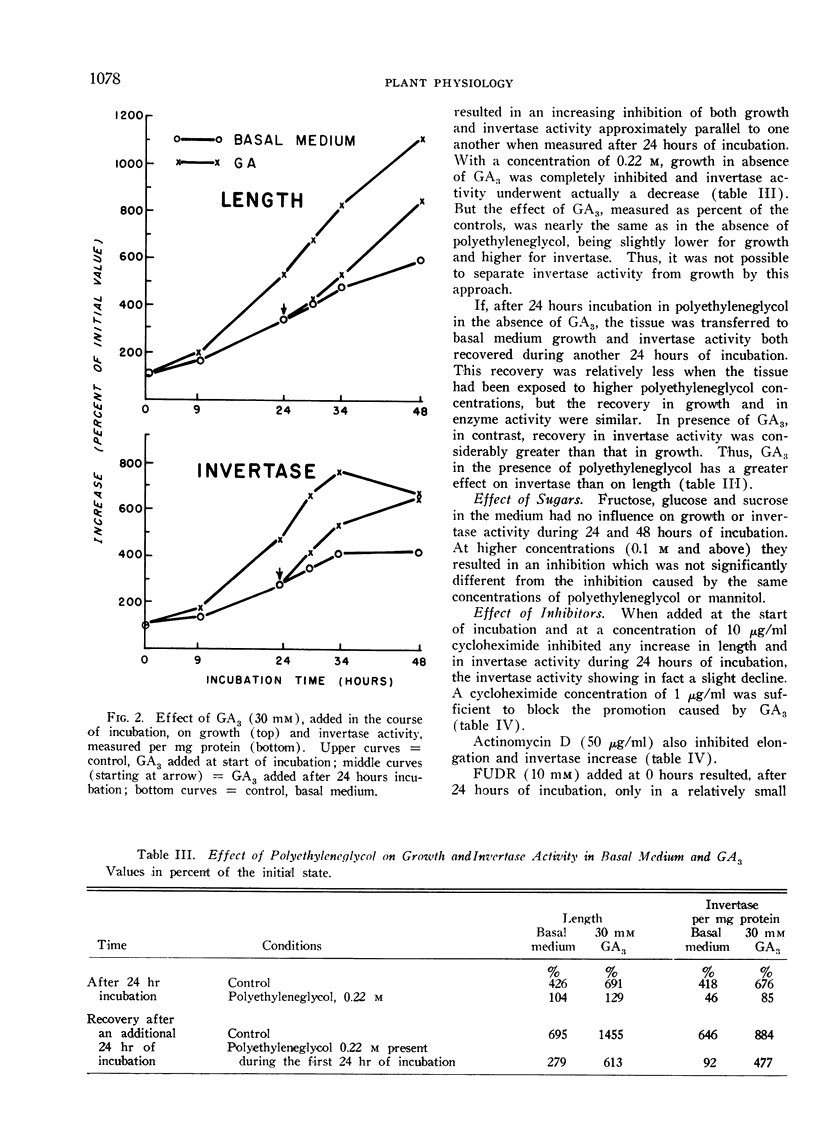
Incubation of the seedlings in solutions of polyethyleneglycol inhibits the increase of both growth and invertase activity, the latter actually undergoing a decline, but causes no great change in the relative effect of GA3. In presence of polyethyleneglycol GA3 has however a relatively greater effect on invertase activity than on growth.
Sugars in the incubation medium have no significant effect on growth and invertase activity in the epicotyl, except inhibition at relatively high concentrations.
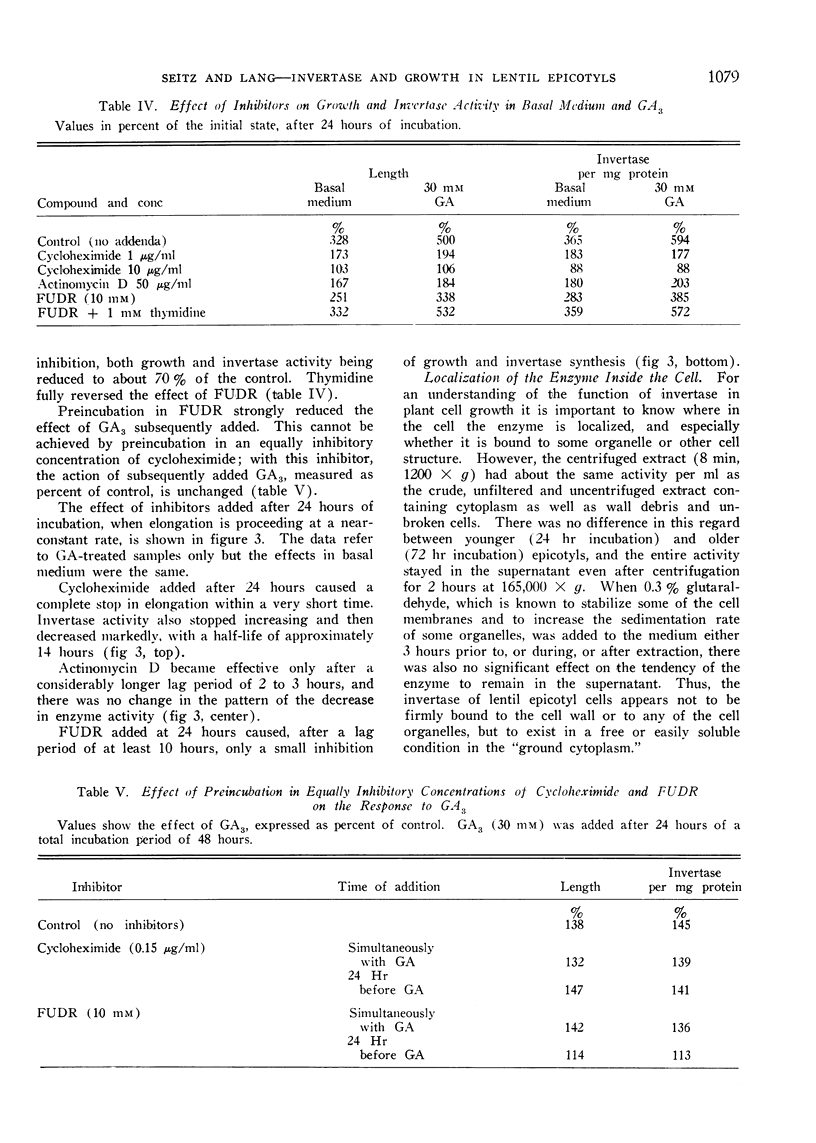
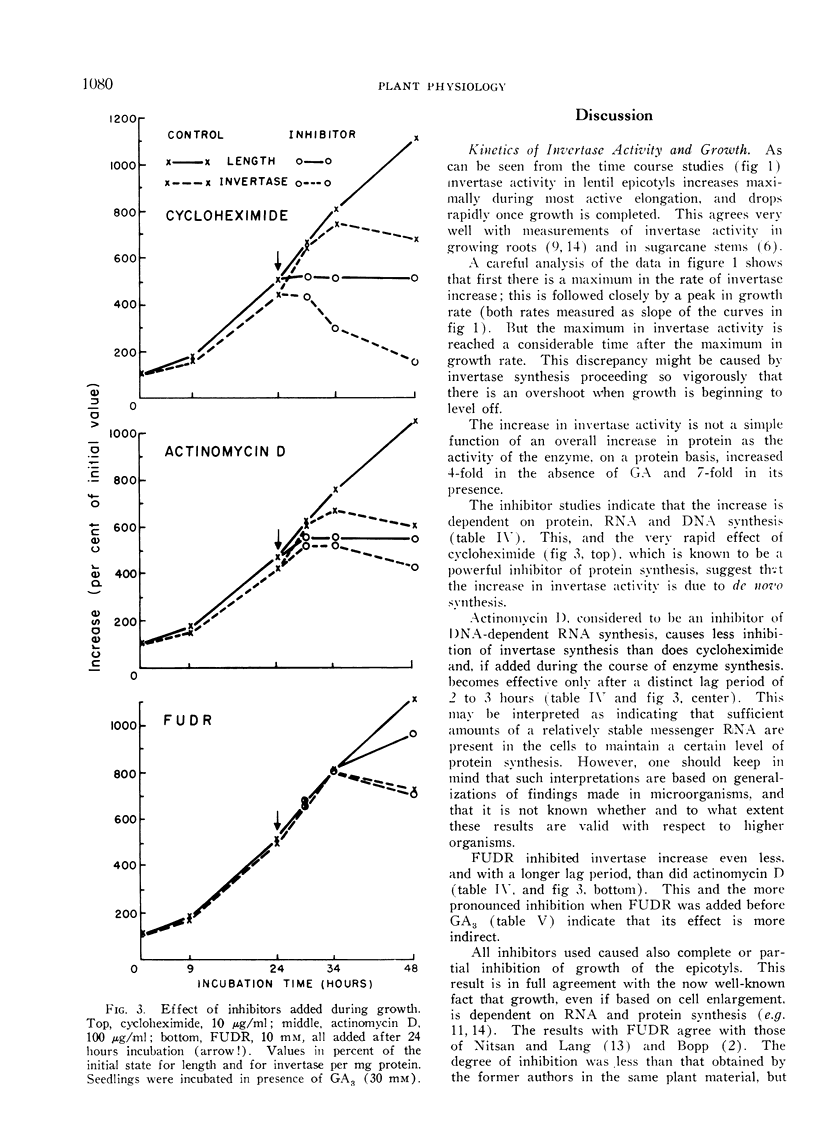
Cycloheximide, actinomycin D, and 5-fluorodeoxyuridine (FUDR) inhibit both growth and the increase in invertase activity. Added during incubation cycloheximide causes complete inhibition of growth and a decrease in invertase activity with no appreciable lag phase. With actinomycin D and FUDR the inhibition occurs after lag periods of 2 to 3 and of at least 10 hours, respectively. Thus the increase in enzyme activity is very probably based on de-novo synthesis, and the enzyme is in a state of turnover during growth.
The enzyme is present in soluble form in the cytoplasm, not firmly bound to any cell structures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON J. S., MACDONALD I. R., KNIGHT A. H. THE DEVELOPMENT OF INVERTASE ACTIVITY IN SLICES OF THE ROOT OF BETA VULGARIS L. WASHED UNDER ASEPTIC CONDITIONS. Biochem J. 1965 Jan;94:175–182. doi: 10.1042/bj0940175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN J., HALL M. A. ENZYME FORMATION IN HIGHER-PLANT TISSUES. DEVELOPMENT OF INVERTASE AND ASCORBATE-OXIDASE ACTIVITIES IN MATURE STORAGE TISSUE OF HELIANTHUS TUBEROSUS L. Biochem J. 1965 May;95:403–410. doi: 10.1042/bj0950403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou K. T., Waldron J. C., Bull T. A. Control of invertase synthesis in sugar cane. Loci of auxin and glucose effects. Plant Physiol. 1966 Feb;41(2):282–288. doi: 10.1104/pp.41.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A., Nitsan J. Relations among cell growth, DNA synthesis, and gibberellin action. Ann N Y Acad Sci. 1967 Aug 9;144(1):180–190. doi: 10.1111/j.1749-6632.1967.tb34012.x. [DOI] [PubMed] [Google Scholar]
- Nitsan J., Lang A. Inhibition of cell division and cell elongation in higher plants by inhibitors of DNA synthesis. Dev Biol. 1965 Dec;12(3):358–376. doi: 10.1016/0012-1606(65)90003-5. [DOI] [PubMed] [Google Scholar]


